Childhood Astrocytomas, Other Gliomas, and Glioneuronal/Neuronal Tumors Treatment (PDQ®): Treatment - Health Professional Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
General Information About Childhood Astrocytomas, Other Gliomas, and Glioneuronal / Neuronal Tumors
Primary brain tumors, including gliomas, are a diverse group of diseases that together constitute the most common solid tumors of childhood. Brain tumors are classified according to histology and molecular features, but tumor location and extent of spread are also important factors that affect treatment and prognosis. Histological features, immunohistochemical analysis, and cytogenetic and molecular genetic findings are used in tumor diagnosis and classification.
Gliomas are thought to arise from neural stem and progenitor cells that are present in the brain and spinal cord. Gliomas are classified on the basis of histological and molecular features, and they represent the most common type of central nervous system (CNS) tumor in children.
Historically, pediatric gliomas were classified into low-grade (World Health Organization [WHO] grades 1–2) and high-grade (WHO grades 3–4) gliomas on the basis of histological features. However, the incorporation of molecular biomarkers has led to a new classification scheme. According to the 2021 WHO Classification of Tumours: Central Nervous System Tumours (5th edition), gliomas, glioneuronal tumors, and neuronal tumors are broadly classified into adult-type diffuse gliomas, pediatric-type diffuse low-grade gliomas, pediatric-type diffuse high-grade gliomas, circumscribed astrocytic gliomas, glioneuronal and neuronal tumors, and ependymal tumors.[1,2] Within these tumor types, various subtypes are recognized, and histological grading ranging from grade 1 to grade 4 is applied to some. Most children with circumscribed astrocytic gliomas, pediatric-type diffuse low-grade gliomas, and glioneuronal and neuronal tumors have a relatively favorable prognosis, especially when a complete surgical resection can be accomplished. Children with pediatric-type diffuse high-grade gliomas generally have a poor prognosis. For information about ependymal tumors, see Childhood Ependymoma Treatment.
The PDQ childhood brain tumor treatment summaries are organized primarily according to the 2021 WHO CNS classification.[1,2]
Anatomy
Childhood gliomas can occur anywhere in the CNS (see Figure 1). For the most common CNS location for each tumor type, see Table 2. 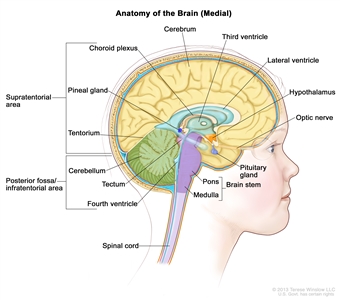
Figure 1. Anatomy of the inside of the brain, showing the cerebrum, cerebellum, brain stem, spinal cord, optic nerve, hypothalamus, and other parts of the brain.
Clinical Features
Presenting symptoms for childhood gliomas depend on the following:
- Anatomical location.
- Size of the tumor.
- Rate of tumor growth.
- Chronological and developmental age of the child.
Infants and young children with circumscribed gliomas (most commonly pilocytic astrocytomas) and, less frequently, diffuse astrocytomas, involving the hypothalamus may present with diencephalic syndrome, which is manifested by failure to thrive in an emaciated, seemingly euphoric child. Such children may have little in the way of other neurological findings, but may present with macrocephaly, intermittent lethargy, and/or visual impairment.[3]
Children with diffuse midline gliomas centered in the pons (previously called diffuse intrinsic pontine gliomas [DIPGs]) may present with the following classic triad of symptoms; however, children may present with only one or two of these symptoms at diagnosis:
- Cranial neuropathies, particularly abducens paresis.
- Long tract signs.
- Ataxia.
Obstructive hydrocephalus caused by expansion of the pons can also be a presenting symptom. Nonspecific symptoms may also occur, including behavioral changes and decreased school performance.
The presentation of circumscribed astrocytomas (e.g., pilocytic astrocytomas) in the brain stem depends on the tumor location. Common presenting symptoms include the following:[4]
- Raised intracranial pressure with associated hydrocephalus.
- Unilateral hemiparesis.
- Unilateral cranial neuropathies.
- Ataxia.
Diagnostic Evaluation
The initial diagnostic evaluation of patients with gliomas includes magnetic resonance imaging (MRI) with and without contrast of the brain and/or spine. The risk of neuraxis dissemination is tumor type dependent, and complete neuraxis imaging, including MRIs of the brain and total spine, may be performed in select patients. In most cases, the specific diagnosis is determined after surgical intervention and pathological classification.
Primary tumors of the brain stem are most often diagnosed on the basis of clinical findings and on neuroimaging studies using MRI, as follows:[5]
- Diffuse midline glioma centered in the pons (DIPG). A presumptive diagnosis of DIPG based on classic imaging and clinical features, in the absence of a histological diagnosis, has been routinely employed. Increasingly however, histological confirmation is obtained for both entry into research studies and molecular characterization of the tumor.[6] Given the technical challenges of pontine biopsies, the procedure is best undertaken by an experienced pediatric neurosurgeon to minimize the risk of irreversible neurological complications.[7,8,9,10,11] Biopsy is recommended for pontine tumors when the diagnosis is uncertain based on imaging findings.
- Non-DIPG brain stem tumors. Biopsy or resection is generally indicated for non-DIPG brain stem tumors.
Lumbar punctures examining the cerebrospinal fluid for circulating tumor cells are not commonly performed in children with these tumor types.
WHO Classification of Childhood CNS Astrocytomas, Gliomas, and Glioneuronal/Neuronal Tumors
The pathological classification of pediatric brain tumors is a highly specialized area that continues to evolve. Rapid advances in molecular genetics have led to major improvements in the accurate diagnosis of brain tumors over the past decade. At the same time, many novel brain tumor entities have been recognized on the basis of unique molecular features. Examination of the diagnostic tissue by an experienced neuropathologist is strongly recommended, along with molecular testing, if available.
According to the 2021 WHO CNS classification, gliomas and glioneuronal/neuronal tumors occurring predominantly in childhood are broadly classified as follows:
- Pediatric-type diffuse high-grade gliomas.
- Pediatric-type diffuse low-grade gliomas.
- Circumscribed astrocytic gliomas.
- Glioneuronal and neuronal tumors.
- Ependymal tumors. For more information, see Childhood Ependymoma Treatment.
Within each tumor type, various subtypes are recognized on the basis of histological and molecular features.
The 2021 WHO CNS classification recommends a layered report structure as follows:[1,2]
- Integrated diagnosis (combined tissue-based histological and molecular diagnosis).
- Histological diagnosis.
- CNS WHO grade.
- Molecular information (listed).
WHO CNS tumor grading
Whereas CNS tumors were previously graded on histopathological grounds and clinical behavior alone (clinicopathological grading), the 2021 WHO CNS grading scheme employs combined histological and molecular grading for many tumor types.[1] Histological grading ranges from 1 to 4, but not all grades are applied to all tumor types, and some tumor types are not graded.
The 2021 WHO CNS classification and grading of the most common types/subtypes of gliomas, glioneuronal tumors, and neuronal tumors (excluding ependymal tumors) occurring in childhood and adolescence are shown in Table 1.
| Tumor Type/Subtype | WHO CNS Grades | |
|---|---|---|
| Pediatric-type diffuse high-grade gliomas: | ||
| Diffuse midline glioma, H3 K27-altered | 4 | |
| Diffuse pediatric-type high-grade glioma, H3-wild type and IDH-wild type | 4 | |
| Infant-type hemispheric glioma | Not assigned | |
| Pediatric-type diffuse low-grade gliomas: | ||
| Diffuse low-grade glioma, MAPK pathway-altered | Not assigned | |
| Diffuse astrocytoma,MYB- orMYBL1-altered | 1 | |
| Circumscribed astrocytic gliomas: | ||
| Pilocytic astrocytoma | 1 | |
| High-grade astrocytoma with piloid features | Not assigned | |
| Pleomorphic xanthoastrocytoma | 2, 3 | |
| Subependymal giant cell astrocytoma | 1 | |
| Glioneuronal and neuronal tumors: | ||
| Ganglioglioma | 1 | |
| Desmoplastic infantile ganglioglioma/desmoplastic infantile astrocytoma | 1 | |
| Dysembryoplastic neuroepithelial tumor | 1 | |
CNS location
Childhood gliomas can occur anywhere in the CNS, although each tumor type tends to occur in specific anatomical locations (see Table 2).
| Tumor Type | Common CNS Location |
|---|---|
| Circumscribed astrocytic gliomas | Cerebellum, optic nerve, optic chiasm/hypothalamus, thalamus and basal ganglia, brain stem, cerebral hemispheres, and spinal cord (rare) |
| Ganglioglioma | Cerebrum, brain stem; occasionally other locations |
| Diffuse midline glioma, H3 K27-altered | Pons, thalamus, spinal cord, and other midline structures |
| Diffuse pediatric-type high-grade glioma, H3-wild type and IDH-wild type | Cerebrum; occasionally other locations |
Cerebellum: More than 80% of gliomas located in the cerebellum are pilocytic astrocytomas (WHO grade 1) and often cystic; most of the remainder represent pediatric-type diffuse low-grade gliomas.[12] High-grade gliomas in the cerebellum are rare.
Brain stem: The term brain stem glioma is a generic description that refers to any tumor of glial origin arising in the brain stem, inclusive of the midbrain, pons, and medulla. While other histologies (e.g., ganglioglioma) can occur in the brain stem, the following two histologies predominate:
- Diffuse midline glioma, H3 K27-altered, which are centered in the pons.[13] These were commonly referred to as diffuse intrinsic pontine gliomas (DIPG) due to their anatomical location. For more information about diffuse midline glioma, H3 K27-altered, see the Genomics of Gliomas, Glioneuronal Tumors, and Neuronal tumors section.
- Pilocytic astrocytomas, which occur throughout the brain stem.
Tumors with exophytic components are overwhelmingly pilocytic astrocytomas.[14] DIPG accounts for approximately 75% to 80% of pediatric brain stem tumors.[15] Most children with DIPGs are diagnosed between the ages of 5 and 10 years. Focal pilocytic astrocytomas in the brain stem occur less frequently.[4]
Optic pathway and hypothalamus: Most tumors arising within the optic pathway (i.e., optic nerve, chiasm, and optic radiations) represent pilocytic astrocytomas, and rarely pediatric-type diffuse low-grade gliomas.[12]
Cerebrum: Most tumors arising in the cerebral hemispheres comprise circumscribed astrocytic gliomas and pediatric-type diffuse low-grade gliomas, followed by pediatric-type diffuse high-grade gliomas.[12]
Genomics of Gliomas, Glioneuronal Tumors, and Neuronal Tumors
Selected cancer susceptibility syndromes associated with pediatric glioma
Neurofibromatosis type 1 (NF1)
Children with NF1 have an increased propensity to develop low-grade gliomas, especially in the optic pathway. Up to 20% of patients with NF1 will develop an optic pathway glioma. Most children with NF1-associated optic nerve gliomas are asymptomatic and/or have nonprogressive symptoms and do not require antitumor treatment. Screening magnetic resonance imaging (MRI) in asymptomatic patients with NF1 is usually not indicated, although some investigators perform baseline MRI for young children who cannot undergo detailed ophthalmologic examinations.[16]
The diagnosis is often based on compatible clinical findings and imaging features. Histological confirmation is rarely needed at the time of diagnosis. When biopsies are performed, these tumors are predominantly pilocytic astrocytomas.[12]
Indications for treatment vary, and are often based on the goal of preserving vision.
Very rarely, patients with NF1 develop high-grade gliomas. Sometimes, this tumor is the result of a transformation of a lower-grade tumor.[17]
Tuberous sclerosis
Patients with tuberous sclerosis have a predilection for developing subependymal giant cell astrocytoma (SEGA). Variants in either TSC1 or TSC2 cause constitutive activation of the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway, leading to increases in proliferation. SEGAs are responsive to molecularly targeted approaches with mTORC1 pathway inhibitors.[18][Level of evidence C2] Patients with tuberous sclerosis are also at risk of developing cortical tubers and subependymal nodules.
Molecular features and recurrent genomic alterations
Recurrent genomic alterations resulting in constitutive activation of the mitogen-activated protein kinase (MAPK) pathway, most commonly involving the BRAF gene, represent the primary (and often sole) oncogenic driver in the vast majority of pediatric low-grade gliomas, including pilocytic/pilomyxoid astrocytomas, gangliogliomas, and others.[12] As a result, most of these tumors are amenable to molecular targeted therapies.
More complex tumor genomes are characteristic of pediatric diffuse high-grade gliomas. These complex genomes include recurrent genomic alterations in the H3 histone encoding genes (e.g., H3F3A, HIST1H3B), DNA damage repair pathways (e.g., TP53, PPM1D, ATM, MDM2), chromatin modifiers (e.g., ATRX, BCOR, SETD2), cell cycle pathways (e.g., CDKN2A, CDKN2B, RB1), and/or oncogene amplifications (PDGFR, VEGFR2, KIT, MYC, MYCN).[19] For most of these tumors, existing conventional and molecular targeted therapies have limited efficacy.
A rare subset of pediatric high-grade gliomas arising in patients with inheritable biallelic mismatch repair deficiency (bMMRD) is characterized by an extraordinarily high mutational burden. Correctly identifying these patients at the time of diagnosis is critical because of intrinsic resistance to temozolomide and responsiveness to treatment with immune checkpoint inhibitors.[20][Level of evidence C3]; [21]
BRAF::KIAA1549
BRAF activation in pilocytic astrocytoma occurs most commonly through a BRAF::KIAA1549 gene fusion, resulting in a fusion protein that lacks the BRAF autoregulatory domain.[22] This fusion is seen in most infratentorial and midline pilocytic astrocytomas, but is present at lower frequency in supratentorial (hemispheric) tumors.[12]
Presence of the BRAF::KIAA1549 fusion is associated with improved clinical outcome (progression-free survival [PFS] and overall survival [OS]) in patients with pilocytic astrocytoma.[23]; [24][Level of evidence C1] Progression to high-grade gliomas is very rare for pediatric gliomas with the BRAF::KIAA1549 fusion.[24]
BRAFvariants
Activating point variants in BRAF, most commonly BRAF V600E, are present in a subset of pediatric gliomas and glioneuronal tumors across a wide spectrum of histologies, including pleomorphic xanthoastrocytoma, pilocytic astrocytoma, ganglioglioma, desmoplastic infantile ganglioglioma/astrocytoma, and others.[12] Some low-grade, infiltrative, pediatric gliomas with an alteration in a MAPK pathway gene, including BRAF, and often resembling diffuse low-grade astrocytoma or oligodendroglioma histologically, are now classified as diffuse low-grade glioma, MAPK pathway altered.[1,25]
Retrospective clinical studies have shown the following:
- In a retrospective series of more than 400 children with low-grade gliomas, 17% of tumors had BRAF V600E variants. The 10-year PFS rate was 27% for patients with BRAF V600E variants, compared with 60% for patients whose tumors did not harbor that variant. Additional factors associated with this poor prognosis included subtotal resection and CDKN2A deletion.[26][Level of evidence C2] Even in patients who underwent a gross-total resection, recurrence was noted in one-third, suggesting that BRAF V600E tumors have a more invasive phenotype than do other low-grade glioma variants.
- In a similar analysis, children with diencephalic low-grade astrocytomas with a BRAF V600E variant had a 5-year PFS rate of 22%, compared with a PFS rate of 52% in children with wild-type BRAF.[27][Level of evidence C2]
- The frequency of the BRAF V600E variant was significantly higher in pediatric low-grade gliomas that transformed to high-grade gliomas (8 of 18 patients) than was the frequency of the variant in tumors that did not transform to high-grade gliomas (10 of 167 cases).[24]
NF1variants
Somatic alterations in NF1 are seen most frequently in children with NF1 and are associated with germline alterations in the tumor suppressor NF1. Loss of heterozygosity for NF1 represents the most common somatic alteration in these patients followed by inactivating variants in the second NF1 allele, and consistent with a second hit required for tumorigenesis. While most NF1 patients with low-grade gliomas have an excellent long-term prognosis, secondary transformation into high-grade glioma may occur in a small subset. Genomically, transformation is associated with the acquisition of additional oncogenic drivers, such as loss of function alterations in CDKN2A, CDKN2B and/or ATRX. Primary high-grade gliomas may also occur in patients with NF1 but are exceedingly rare. Genomic alterations involving the MAPK signaling pathway other than NF1 are very uncommon in gliomas occurring in children with NF1.[17]
ALK,NTRK1,NTRK2,NTRK3, orROS1gene fusions
High-grade gliomas with distinctive molecular characteristics arise in infants, typically in those diagnosed during the first year of life.[28,29,30] These tumors are characterized by recurrent oncogenic gene fusions involving ALK, NTRK1, NTRK2, NTRK3, or ROS1 as the primary and, typically, sole oncogenic driver. Infants with this type of glioma, now classified as infant-type hemispheric glioma, have a much better prognosis compared with older children with high-grade gliomas. Remarkably, these tumors may evolve from high-grade to low-grade histology over time, and it remains unclear how much this phenomenon is a consequence of natural disease history versus treatment-induced changes.[28]
ROS1 gene fusions have also been reported in gliomas occurring in older children and adults. A retrospective meta-analysis that included 40 children older than 1 year revealed that ROS1 gene fusions occurred in diverse glioma histologies, including diffuse high-grade and low-grade gliomas and glioneuronal tumors.[30] Similar to ROS1-altered cases occurring in infants, tumor variants in other known driver genes were rare. However, tumor copy number alterations were more frequent in older children than infants.
Other genomic alterations
As an alternative to BRAF activation or NF1 loss, other primary oncogenic driver alterations along the MAPK signaling pathway have been observed in pilocytic astrocytomas and other pediatric-type gliomas. These include oncogenic variants and/or fusions involving FGFR1, FGFR2, PTPN11, RAF1,NTRK2, and others.[12,31,32]
Low-grade gliomas with rearrangements in the MYB family of transcription factors [12,33,34] have now been classified as a separate entity: diffuse astrocytoma, MYB- or MYBL1-altered, WHO grade 1.[1]
Angiocentric gliomas
Angiocentric gliomas typically arise in children and young adults as cerebral tumors presenting with seizures.[35]
Two reports in 2016 identified MYB gene alterations as being present in almost all cases diagnosed as angiocentric glioma, with QKI being the primary fusion partner in cases where fusion-partner testing was possible.[32,36] While angiocentric gliomas most commonly occur supratentorially, brain stem angiocentric gliomas with MYB::QKI fusions have also been reported.[37,38]
Astroblastomas,MN1-altered
Astroblastomas are defined histologically as glial neoplasms composed of GFAP-positive cells and contain astroblastic pseudorosettes that often demonstrate sclerosis. Astroblastomas are diagnosed primarily in childhood through young adulthood.[35]
The following studies have described genomic alterations associated with astroblastoma:
- A report describing a molecular classification of CNS primitive neuroectodermal tumors (PNETs) identified an entity called CNS high-grade neuroepithelial tumor with MN1 alteration (CNS HGNET-MN1) that was characterized by gene fusions involving MN1.[39] Most tumors with a histological diagnosis of astroblastoma (16 of 23) belonged to this molecularly defined entity.
- A report of 27 histologically defined astroblastomas found that 10 cases had MN1 rearrangements, 7 cases had BRAF rearrangements, and 2 cases had RELA rearrangements.[40] Methylation array analysis showed that the cases with MN1 rearrangements clustered with CNS HGNET-MN1, the BRAF-altered cases clustered with pleomorphic xanthoastrocytomas, and the RELA cases clustered with ependymomas.
- Genomic evaluation of eight cases of astroblastoma identified four with MN1 alterations. Of the remaining four cases, two had genomic alterations consistent with high-grade glioma and two cases could not be classified on the basis of their molecular characteristics.[41]
- One study described eight cases of astroblastoma. All five cases that underwent fluorescence in situ hybridization analysis showed MN1 rearrangements.[42]
These reports suggest that the histological diagnosis of astroblastoma encompasses a heterogeneous group of genomically defined entities. Astroblastomas with MN1 fusions represent a distinctive subset of histologically diagnosed cases.[43]
IDH1andIDH2variants
IDH1- and IDH2-altered tumors occur in the pediatric population as low-grade gliomas (WHO Grade 2), high-grade gliomas (WHO Grade 3 and 4), and oligodendrogliomas with codeletion of 1p and 19q. For more information about IDH1- and IDH2-altered gliomas, see the IDH1 and IDH2 variants section in the Molecular features of pediatric-type high-grade gliomas section.
Molecular features of pediatric-type high-grade gliomas
Pediatric high-grade gliomas are biologically distinct from those arising in adults.[44,45,46,47]
Subgroups identified using DNA methylation patterns
Pediatric-type high-grade gliomas can be separated into distinct subgroups on the basis of epigenetic patterns (DNA methylation). These subgroups show distinguishing chromosome copy number gains/losses and gene variants in the tumor.[19,48,49] Particularly distinctive subtypes of pediatric high-grade gliomas are those with recurring variants at specific amino acids in histone genes, and together these account for approximately one-half of pediatric high-grade gliomas.[19]
The following pediatric-type high-grade glioma subgroups were identified on the basis of their DNA methylation patterns, and they show distinctive molecular and clinical characteristics:[19]
Genomic alterations associated with diffuse midline gliomas
The histone K27 variants: H3.3 (H3F3A) and H3.1 (HIST1H3Band, rarely,HIST1H3C) variants at K27 and EZHIP
The histone K27–altered cases occur predominantly in middle childhood (median age, approximately 10 years), are almost exclusively midline (thalamus, brain stem, and spinal cord), and carry a very poor prognosis. The 2021 WHO classification groups these cancers into a single entity: diffuse midline glioma, H3 K27-altered. However, there are clinical and biological distinctions between cases with H3.3 and H3.1 variants, as described below.[1]
Diffuse midline glioma, H3 K27-altered, is defined by loss of H3 K27 trimethylation either due to an H3 K27M variant or, less commonly, overexpression of EZHIP. This entity includes most high-grade gliomas located in the thalamus, pons (diffuse intrinsic pontine gliomas [DIPGs]), and spinal cord, predominantly in children, but also in adults.[50]
H3.3 K27M: H3.3 K27M cases occur throughout the midline and pons, account for approximately 60% of cases in these locations, and commonly present between the ages of 5 and 10 years.[19] The prognosis for H3.3 K27M patients is especially poor, with a median survival of less than 1 year; the 2-year survival rate is less than 5%.[19] Leptomeningeal dissemination is frequently observed in H3.3 K27M patients.[51]
H3.1 K27M: H3.1 K27M cases are approximately fivefold less common than H3.3 K27M cases. They occur primarily in the pons and present at a younger age than other H3.3 K27M patients (median age, 5 years vs. 6–10 years). These patients have a slightly more favorable prognosis than do H3.3 K27M patients (median survival, 15 months vs. 11 months). Variants in ACVR1, which is also the variant observed in the genetic condition fibrodysplasia ossificans progressiva, are present in a high proportion of H3.1 K27M cases.[19,52,53]
H3.2 K27M: Rarely, K27M variants are also identified in H3.2 (HIST2H3C) cases.[19]
A subset of tumors with H3 K27 variants will have a BRAF V600E or FGFR1 co-variant. A retrospective cohort of 29 tumors combined with 31 cases previously reported in the literature demonstrated a somewhat higher propensity for a thalamic location. These cases exhibit a unique DNA methylation cluster that is distinct from other diffuse midline glioma subgroups and glioma subtypes with BRAF or FGFR1 alterations. The median survival for these patients exceeded 3 years.[54] A separate retrospective study of pediatric and adult patients with H3 K27-altered gliomas revealed BRAF V600E variants in 5.8% (9 of 156) and FGFR1 variants in 10.9% (17 of 156) of patients younger than 20 years.[55] Other recurrent genetic alterations detected in pediatric patients included variants in TP53, ATRX, PIK3CA, and amplifications of PDGFRA and KIT. FGFR1 variants were noted to be more frequent in patients older than 20 years (31.8%, 47 of 148).
EZHIP overexpression: The small minority of patients with diffuse midline gliomas lacking histone H3 variants often show EZHIP overexpression.[50] EZHIP inhibits PRC2 activity, leading to the same loss of H3 K27 trimethylation that is induced by H3 K27M variants.[56] Overexpression of EZHIP is likewise observed in posterior fossa type A ependymomas, which also shows loss of H3 K27 methylation.[57]
H3.3 (H3F3A) variant at G34
The H3.3 G34 subtype arises from H3.3 glycine 34 to arginine/valine (G34R/V) variants.[48,49] This subtype presents in older children and young adults (median age, 14–18 years) and arises exclusively in the cerebral cortex.[48,49] H3.3 G34 cases commonly have variants in TP53 and ATRX (95% and 84% of cases, respectively, in one large series) and show widespread hypomethylation across the whole genome. In a series of 95 patients with the H3.3 G34 subtype, 44% of patients also had a variant in PDGFRA at the time of diagnosis, and 81% of patients had PDGFRA variants observed at relapse.[58]
Patients with H3F3A variants are at high risk of treatment failure,[59] but the prognosis is not as poor as that of patients with histone 3.1 or 3.3 K27M variants.[49] O-6-methylguanine-DNA methyltransferase (MGMT) methylation is observed in approximately two-thirds of cases, and aside from the IDH1-altered subtype (see below), the H3.3 G34 subtype is the only pediatric high-grade glioma subtype that demonstrates MGMT methylation rates exceeding 20%.[19]
IDH1andIDH2variants
IDH1- and IDH2-altered tumors occur in the pediatric population as low-grade gliomas (WHO grade 2), high-grade gliomas (WHO grades 3 and 4), and oligodendrogliomas with codeletion of 1p and 19q.[60]
- IDH1 variants are much more common than IDH2 variants, accounting for approximately 90% of pediatric IDH-altered CNS tumors.
- IDH-altered low-grade gliomas are more common than IDH-altered high-grade gliomas, accounting for approximately three-fourths of IDH-altered pediatric glioma cases.
- Oligodendrogliomas with IDH variants represent approximately 20% of pediatric CNS tumors with IDH variants.
- The median age at diagnosis for pediatric patients with IDH-altered tumors is approximately 16 years, and IDH-altered CNS tumors are very uncommon in children aged 10 years and younger.
- Like astrocytomas with IDH variants in adults, those in affected children commonly have TP53 variants (approximately 90% of cases) and ATRX variants (approximately 50%).
- Like IDH-altered, low-grade gliomas in adults, low-grade tumors in pediatric patients can also show progression to high-grade gliomas.
IDH1-altered cases represent a small percentage of high-grade gliomas (approximately 5%–10%) seen in pediatrics, and are almost exclusively older adolescents (median age in a pediatric population, 16 years) with hemispheric tumors.[19,60] These tumors are classified under adult-type diffuse glioma, as astrocytoma, IDH-altered in the 2021 WHO CNS classification. IDH1-altered cases often show TP53 variants, MGMT promoter methylation, and a glioma-CpG island methylator phenotype (G-CIMP).[48,49]
Pediatric patients with IDH1 variants have a more favorable prognosis than patients with other types of high-grade gliomas.[19] A retrospective multi-institutional review of pediatric patients with IDH-altered gliomas and available outcome data (n = 76) reported a 5-year PFS rate of 44% (95% CI, 25%–59%) and a 5-year OS rate of 92% (95% CI, 79%–97%).[60] Approximately 25% of the gliomas in the cohort were classified as high grade. There was no difference in 5-year PFS rates observed between tumor grades. However, patients with high-grade tumors had a worse 5-year OS rate of 75% (95% CI, 40%–91%).
Rare, IDH-altered, high-grade gliomas have been reported to occur in children with mismatch repair–deficiency syndromes (Lynch syndrome or constitutional mismatch repair deficiency syndrome).[61] These tumors, termed primary mismatch repair–deficient IDH-altered astrocytomas (PMMRDIAs), could be distinguished from other IDH-altered gliomas by methylation profiling. PMMRDIAs have molecular features that are distinct from most IDH-altered gliomas, including a hypervariant phenotype and frequent activation of receptor tyrosine kinase pathways. Patients with PMMRDIAs have a markedly worse prognosis than patients with other IDH-altered gliomas, with a median survival of 15 months.
Pleomorphic xanthoastrocytoma (PXA)–like
Approximately 10% of pediatric high-grade gliomas have DNA methylation patterns that are PXA-like.[49] PXA-like cases commonly have BRAF V600E variants and a relatively favorable outcome (approximately 50% survival at 5 years).[19,59]
High-grade astrocytoma with piloid features
This entity was included in the 2016 WHO classification (called pilocytic astrocytoma with anaplasia) to describe tumors with histological features of pilocytic astrocytoma, increased mitotic activity, and additional high-grade features. The current nomenclature was adopted in the 2021 WHO classification. A more recent publication described a cohort of 83 cases with these histological features (referred to as anaplastic astrocytoma with piloid features) that shared a common DNA methylation profile, which is distinct from the methylation profiles of other gliomas. These tumors occurred more often in adults (median age, 41 years), and they harbored frequent deletions of CDKN2A/B, MAPK pathway alterations (most often in the NF1 gene), and variants or deletions of ATRX. They are associated with a clinical course that is intermediate between pilocytic astrocytoma and IDH–wild-type glioblastoma.[62]
Other variants
Pediatric patients with glioblastoma multiforme high-grade glioma whose tumors lack both histone variants and IDH1 variants represent approximately 40% of pediatric glioblastoma multiforme cases.[19,63] This is a heterogeneous group, with higher rates of gene amplifications than other pediatric high-grade glioma subtypes. The most commonly amplified genes are PDGFRA, EGFR, CCND/CDK, and MYC/MYCN.[48,49] MGMT promoter methylation rates are low in this group.[63] One report divided this group into three subtypes. The subtype characterized by high rates of MYCN amplification showed the poorest prognosis, while the subtype characterized by TERT promoter variants and EGFR amplification showed the most favorable prognosis. The third group was characterized by PDGFRA amplification.[63]
High-grade gliomas in infants
Infants and young children with high-grade gliomas appear to have tumors with distinctive molecular characteristics [28,29] when compared with tumors of older children and adults with high-grade gliomas. An indication of this difference was noted with the application of DNA methylation analysis to pediatric high-grade tumors, which found that approximately 7% of pediatric patients with a histological diagnosis of high-grade glioma had tumors with methylation patterns more closely resembling those of low-grade gliomas.[19] Ten of 16 infants (younger than 1 year) with a high-grade glioma diagnosis were in this methylation array–defined group.[19] The 5-year survival rate for patients in this report diagnosed at younger than 1 year exceeded 60%, while the 5-year survival rate for patients aged 1 to 3 years and older was less than 20%.
Two studies of the molecular characteristics of high-grade gliomas in infants and young children have further defined the distinctive nature of tumors arising in children younger than 1 year. A key finding from both studies is the importance of gene fusions involving tyrosine kinases (e.g., ALK, NTRK1, NTRK2, NTRK3, and ROS1) in patients in this age group. Both studies also found that infants with high-grade gliomas whose tumors have these gene fusions have survival rates much higher than those of older children with high-grade gliomas.[28,29]
The first study presented data for 118 children younger than 1 year with a low-grade or high-grade glioma diagnosis who had tumor tissue available for genomic characterization.[28] Approximately 75% of the cases were classified as low grade, but the diminished utility of histological classification in this age group was illustrated by the relatively low OS rate for the low-grade cohort (71%) and the relatively favorable survival for the high-grade cohort (55%). Rates of surgical resection were higher for patients with high-grade tumors, a result of many of the low-grade tumors occurring in midline locations while the high-grade tumors were found in supratentorial locations. This finding may also help to explain the relative outcomes for the two groups. Genomic characterization divided the infant glioma population into the following three groups, the first of which included patients with high-grade gliomas:
- Group 1 tumors were receptor tyrosine kinase driven and primarily high grade (83%). These tumors harbored lesions in ALK, ROS1, NTRK, and MET. The median age at diagnosis was 3 months, and OS rates were approximately 60%.
- Group 2 tumors were RAS/MAPK driven and were all hemispheric low-grade gliomas, representing one-fourth of hemispheric gliomas in infants. BRAF V600E was the most common alteration, followed by FGFR1 alterations and BRAF fusions. This group had a median age at presentation of 8 months and had the most favorable outcome (10-year OS rate, 93%).
- Group 3 tumors were RAS/MAPK driven with low-grade histology and midline presentation (approximately 80% optic pathway/hypothalamic gliomas). Most group 3 tumors showed either BRAF fusions or BRAF V600E. Median age at diagnosis was 7.5 months. The 5-year progression-free survival (PFS) rate was approximately 20%, and the 10-year OS rate was approximately 50% (far inferior to that of optic pathway/hypothalamic gliomas in children aged >1 year).
The second study focused on tumors from children younger than 4 years with a pathological diagnosis of WHO grades 2, 3, and 4 gliomas, astrocytomas, or glioneuronal tumors. Among the 191 tumors studied that met inclusion criteria, 61 had methylation profiles consistent with glioma subtypes that occur in older children (e.g., IDH1, diffuse midline glioma H3 K27-altered, SEGA, pleomorphic xanthoastrocytoma, etc.). The remaining 130 cases were called the intrinsic set and were the focus of additional molecular characterization:[29]
- The intrinsic set contained most of the patients diagnosed before age 1 year (49 of 63 patients, 78%) and had a median age of 7.2 months. Tumors were frequently in a superficial hemispheric location, often involving the meninges, and had a well-defined border with adjacent normal brain.
- The methylation classifier placed most of these cases in either the desmoplastic infantile ganglioglioma/astrocytoma (DIG/DIA) subgroup or in the infantile hemispheric glioma subgroup.
- For 41 tumors from the intrinsic set in which tissue was available for gene panel and RNA sequencing, 25 tumors had fusions involving either ALK (n = 10), NTRK1 (n = 2), NTRK2 (n = 2), NTRK3 (n = 8), ROS1 (n = 2), or MET (n = 1). BRAF variants (n = 3) were observed in cases that were high scoring by methylation array for the DIG/DIA or DIG/DIA-like subgroups.
- For patients in the intrinsic set, the 5-year survival rate was higher for patients whose tumors had gene fusions when compared with patients whose tumors lacked fusions (approximately 80% vs. 60%, respectively). However, both of these groups of patients had much higher survival rates than other children with high-grade gliomas.
Secondary high-grade glioma
Childhood secondary high-grade glioma (high-grade glioma that is preceded by a low-grade glioma) is uncommon (2.9% in a study of 886 patients). No pediatric low-grade gliomas with the BRAF::KIAA1549 fusion transformed to a high-grade glioma, whereas low-grade gliomas with the BRAF V600E variants were associated with increased risk of transformation. Seven of 18 patients (approximately 40%) with secondary high-grade glioma had BRAF V600E variants, with CDKN2A alterations present in 8 of 14 cases (57%).[24]
Molecular features of glioneuronal and neuronal tumors
Glioneuronal and neuronal tumors are generally low-grade tumors. Select histologies recognized by the 2021 WHO classification include the following:[1]
- Ganglioglioma.
- Desmoplastic infantile ganglioglioma/desmoplastic infantile astrocytoma.
- Dysembryoplastic neuroepithelial tumor.
- Papillary glioneuronal tumor.
- Rosette-forming glioneuronal tumor.
- Dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease).
- Gangliocytoma.
- Diffuse leptomeningeal glioneuronal tumor.
- Central neurocytoma.
- Extraventricular neurocytoma.
Ganglioglioma
Ganglioglioma presents during childhood and into adulthood. It most commonly arises in the cerebral cortex and is associated with seizures, but it also presents in other sites, including the spinal cord.[64,65]
The unifying theme for the molecular pathogenesis of ganglioglioma is genomic alterations leading to MAPK pathway activation.[32,66]BRAF alterations are observed in approximately 50% of ganglioglioma cases, with V600E being by far the most common alteration. However, other BRAF variants and gene fusions are also observed. Other less commonly altered genes in ganglioglioma include KRAS, FGFR1, FGFR2, RAF1, NTRK2, and NF1.[32,66]
Desmoplastic infantile astrocytomas (DIA) and desmoplastic infantile gangliogliomas (DIG)
DIA and DIG most often present in the first year of life and show a characteristic imaging appearance in which a contrast-enhancing solid nodule accompanies a large cystic component.[67,68] DIG is more common than DIA,[67] and by methylation array analysis, both diagnoses cluster together.[69] Survival outcome is generally favorable with surgical resection.[67]
The most commonly observed genomic alterations in DIA and DIG are BRAF variants involving V600. Gene fusions involving kinase genes are observed less frequently.
- Among 16 cases confirmed by histology and DNA methylation profiling to be DIA and DIG, BRAF variants were observed in seven cases (43.8%): four BRAF V600E variants and three BRAF V600D variants.[69] One additional case had an EML4::ALK fusion. BRAF variants were present in 4 of 12 DIG cases (25%) (with 3 of 4 altered cases having BRAF V600D) and in 3 of 4 DIA cases (75%) (all 3 altered cases with BRAF V600E).
- One study of seven DIG cases found MAPK pathway alterations in four (57%).[70] Three alterations involved BRAF (V600E, V600D, and one deletion/insertion centered at V600) and one was a TPM3::NTRK1 in-frame fusion. Notably, the variant allele frequency was low (8%–27%), suggesting that DIG is characterized by a prominent nonneoplastic component resulting in low clonal driver variant allele frequencies.
- Another report also described the BRAF V600D variant in a DIG case.[71] As the V600D variant is far less common than V600E in other cancers, its detection in multiple DIG cases suggests an association between the variant and DIG.
Dysembryoplastic neuroepithelial tumor (DNET)
DNET presents in children and adults, with the median age at diagnosis in mid-to-late adolescence. It is characterized histopathologically by the presence of columns of oligodendroglial-like cells and cortical ganglion cells floating in mucin.[72] The temporal lobe is the most common location, and it is associated with drug-refractory epilepsy.[65,73]
FGFR1 alterations have been reported in 60% to 80% of DNETs, and include FGFR1 activating point variants, internal tandem duplication of the kinase domain, and activating gene fusions.[32,74,75]BRAF variants are uncommon in DNET.
Papillary glioneuronal tumor
Papillary glioneuronal tumor is a low-grade biphasic neoplasm with astrocytic and neuronal differentiation that primarily arises in the supratentorial compartment.[35] The median age at presentation is in the early 20s, but it can be observed during childhood through adulthood.
The primary genomic alteration associated with papillary glioneuronal tumor is a gene fusion, SLC44A1::PRKCA, that is associated with the t(9:17)(q31;q24) translocation.[76,77] In one study of 28 cases diagnosed histologically as papillary glioneuronal tumor using methylation arrays, 11 of the cases clustered in a distinctive methylation class, while the remaining cases showed methylation profiles typical for other tumor entities. Molecular analysis of the cases in the distinctive methylation cluster showed that all of them had the SLC44A1::PRKCA gene fusion except for a single case with a NOTCH1::PRKCA gene fusion.[78] This suggests that molecular methods for identifying the presence of a PRKCA fusion are less susceptible to misclassification in diagnosing papillary glioneuronal tumor than are morphology-based methods.
Rosette-forming glioneuronal tumor (RGNT)
RGNT presents in adolescents and adults, with tumors generally located infratentorially, although tumors can arise in mesencephalic or diencephalic regions.[79] The typical histological appearance shows both a glial component and a neurocytic component arranged in rosettes or perivascular pseudorosettes.[35] Outcome for patients with RGNT is generally favorable, consistent with the WHO grade 1 designation.[79]
DNA methylation profiling shows that RGNT has a distinct epigenetic profile that distinguishes it from other low-grade glial/glioneuronal tumor entities.[79] A study of 30 cases of RGNT observed FGFR1 hotspot variants in all analyzed tumors.[79] In addition, PIK3CA activating variants were concurrently observed in 19 of 30 cases (63%). Missense or damaging variants in NF1 were identified in 10 of 30 cases (33%), with 7 tumors having variants in FGFR1, PIK3CA, and NF1. The co-occurrence of variants that activate both the MAPK pathway and the PI3K pathway makes the variant profile of RGNT distinctive among astrocytic and glioneuronal tumors.
Diffuse leptomeningeal glioneuronal tumor (DLGNT)
DLGNT is a rare CNS tumor that has been characterized radiographically by leptomeningeal enhancement on MRI that may involve the posterior fossa, brain stem region, and spinal cord.[80] Intraparenchymal lesions, when present, typically involve the spinal cord.[80] Localized intramedullary glioneuronal tumors without leptomeningeal dissemination and with histomorphological, immunophenotypic, and genomic characteristics similar to DLGNT have been reported.[81]
DLGNT showed a distinctive epigenetic profile on DNA methylation arrays, and unsupervised clustering of array data applied to 30 cases defined two subclasses of DLGNT: methylation class (MC)-1 (n = 17) and MC-2 (n = 13).[80] Of note, many of the array-defined cases had originally been diagnosed as other entities (e.g., primitive neuroectodermal tumors, pilocytic astrocytoma, and anaplastic astrocytoma). Patients with DLGNT-MC-1 were diagnosed at an earlier age than were patients with DLGNT-MC-2 (5 years vs. 14 years, respectively). The 5-year OS rate was higher for patients with DLGNT-MC-1 than for those with DLGNT-MC-2 (100% vs. 43%, respectively). Genomic findings from the 30 cases of methylation array–defined DLGNT are provided below:
- All 30 cases showed loss of chromosome 1p, but only 6 of 17 DLGNT-MC-1 cases showed additional gain of chromosome 1q, compared with all cases of DLGNT-MC-2.[80] A separate report found that chromosome 1q gain was an adverse prognostic factor in patients with DLGNT (including cases with localized disease),[82] which is consistent with the inferior outcome for patients with DLGNT-MC-2.
- Co-deletions of 1p/19q were more frequent in the DLGNT-MC-1 group (7 of 13, 54%) than in the DLGNT-MC-2 group (2 of 13, 15%). In contrast to oligodendroglioma, variants of IDH1 and IDH2 were not identified.[80]
- MAPK pathway activation is common in DLGNT cases.[80] The KIAA1549::BRAF fusion was present in 11 of 15 DLGNT-MC-1 cases (65%) and in 9 of 13 DLGNT-MC-2 cases (69%). Fusions involving NTRK1, NTRK2, or NTRK3 were present in one case each, and another case had a TRIM33::RAF1 fusion.
Extraventricular neurocytoma
Extraventricular neurocytoma is histologically similar to central neurocytoma, consisting of small uniform cells that demonstrate neuronal differentiation. However, extraventricular neurocytoma arises in the brain parenchyma rather than in association with the ventricular system.[35] It presents during childhood through adulthood.
In a study of 40 tumors histologically classified as extraventricular neurocytoma and subjected to methylation array analysis, only 26 formed a separate cluster distinctive from reference tumors of other histologies.[83] Among cases with an extraventricular neurocytoma methylation array classification for which genomic characterization could be performed, 11 of 15 (73%) showed rearrangements affecting members of the FGFR family, with FGFR1::TACC1 being the most common alteration.[83]
Prognosis
Circumscribed astrocytic gliomas, pediatric-type diffuse low-grade gliomas, and glioneuronal/neuronal tumors
These tumors generally carry a relatively favorable prognosis, particularly for well-circumscribed lesions where a radical resection may be possible.[84,85] With the exception of diffuse leptomeningeal glioneuronal tumors, disseminated or multifocal disease is rare.[86]
Unfavorable clinical prognostic features include the following:[87,88,89]
- Young age.
- Inability to obtain a complete resection.
- Diencephalic syndrome.
- Disseminated or multifocal disease. When disseminated or multifocal disease is present, it is associated with a poorer long-term outcome.
On a molecular level, presence of a BRAF V600E variant, especially in conjunction with a CDKN2A or CDKN2B homozygous deletion, has been recognized as a negative prognostic factor, with risk of transformation to a higher-grade tumor. Conversely, the presence of a BRAF::KIAA1549 fusion confers a better clinical outcome in patients with circumscribed astrocytic gliomas.[26][Level of evidence C2]
In children with tumors of the visual pathway, both visual outcomes and clinical assessments are important. Children with isolated optic nerve tumors have a better prognosis than do children with lesions that involve the chiasm or that extend along the optic pathway.[90,91]; [92][Level of evidence C1] Children with NF1 also have a better prognosis, especially when the tumor is found in asymptomatic patients.[93] Better visual acuity at diagnosis, older age at diagnosis, and presence of NF1 are associated with better visual outcomes.[94]
Pediatric-type diffuse high-grade gliomas
These tumors carry a very poor prognosis with currently available therapies.
Patients with diffuse midline glioma, H3 K27-altered have the poorest prognosis, with 3-year survival rates below 5%.[49]
Diffuse brain stem tumors
The following definitions of brain stem tumors are used:
- Brain stem glioma: A general term describing an astrocytoma arising in the brain stem. Such tumors can be circumscribed or diffuse and can occur in any location in the brain stem, including the midbrain, pons, and medulla.
- Diffuse intrinsic pontine glioma (DIPG): A term used to describe an infiltrating astrocytoma (presumed diffuse midline glioma) centered in the pons.
- Diffuse midline glioma, H3 K27-altered: The pathological diagnosis of most tumors that present with imaging features consistent with a DIPG.
The median survival for children with DIPGs is less than 1 year, although about 10% of children will survive longer than 2 years.[95,96] In contrast, patients with focal astrocytomas (e.g., pilocytic astrocytomas) have a markedly improved prognosis, with 5-year OS rates exceeding 90%.[4]
One report from a clinical trial included 42 children and adolescents with newly diagnosed midline thalamic high-grade gliomas. The study found that tumor location, enhancement pattern, diffusion restriction, and variant status did not significantly affect survival.[97] Leptomeningeal metastatic dissemination and lower surgical resection rates were associated with poorer outcomes.
Prognostic factors include the following:
- Histology/grade of the tumor: Astrocytic tumors predominate in the brain stem. WHO grade 1 tumors (e.g., pilocytic astrocytomas and gangliogliomas) have a favorable prognosis and can arise throughout the brain stem, including the tectum of the midbrain, focally within the pons, or at the cervicomedullary junction where they are often exophytic. Low-grade diffuse astrocytomas (WHO grade 2) occurring outside the pons in other brain stem locations tend to be tumors with a more favorable prognosis.[98]
DIPGs are diffuse astrocytomas that, when biopsied at diagnosis, can range from diffuse astrocytomas (WHO grade 2) to glioblastomas (WHO grade 4). At postmortem evaluation, DIPGs are also generally anaplastic astrocytomas (WHO grade 3) or glioblastomas (WHO grade 4) by morphological criteria, although WHO grade 2 regions can also be identified.[52,53,99,100,101]
Approximately 80% of DIPGs, regardless of histological grade, demonstrate a histone H3.3 or H3.1 variant and are now classified by the WHO as diffuse midline gliomas, H3 K27M-altered. All diffuse midline gliomas, H3 K27M-altered, are WHO grade 4, regardless of histological grade, reflecting the poor prognosis of children with this diagnosis.
- Age at diagnosis: Slightly prolonged survival has been found in those either very young (≤3 years) or older (≥10 years) at diagnosis. Approximately 4% of children with DIPGs are diagnosed when younger than 3 years. The prognosis of these children is less dismal than that of older children, with 28% of younger children alive at 2 years compared with 8% of children aged 3 to 10 years at diagnosis and 14% of children older than 10 years at diagnosis. For children aged 10 years and older, long-term survival was associated with older age at presentation and a longer duration of symptoms.[102] The more favorable prognosis for young children may reflect the presence of different biological characteristics in different age groups.[95,103]
- NF1: Children with NF1 and brain stem gliomas may have a better prognosis than other patients who have intrinsic lesions.[104,105]
- Clinical and imaging features present at diagnosis: For children with DIPGs, features associated with surviving less than 2 years include the presence at diagnosis of cranial nerve palsies, ring enhancement, necrosis, and extrapontine extension.[95] The 2-year survival rate is less than 10% for patients with these characteristics.
- Duration of symptoms at diagnosis: Longer duration of symptoms is associated with a more favorable prognosis. The 2-year survival rates range from 7% for patients with duration of symptoms less than 6 months to 29% for patients with duration of symptoms of 24 months or longer.[95]
- Histone variants: Patients with H3.1 K27M variants have a longer median survival (15 months) than do patients with H3.3 K27M variants (10.4 months) or patients without a histone variant (10.5 months).[95]
References:
- Louis DN, Perry A, Wesseling P, et al.: The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23 (8): 1231-1251, 2021.
- WHO Classification of Tumours Editorial Board, ed.: WHO Classification of Tumours: Central Nervous System Tumours. Vol. 6. 5th ed. IARC Press; 2021.
- Kilday JP, Bartels U, Huang A, et al.: Favorable survival and metabolic outcome for children with diencephalic syndrome using a radiation-sparing approach. J Neurooncol 116 (1): 195-204, 2014.
- Klimo P, Pai Panandiker AS, Thompson CJ, et al.: Management and outcome of focal low-grade brainstem tumors in pediatric patients: the St. Jude experience. J Neurosurg Pediatr 11 (3): 274-81, 2013.
- Liu AK, Brandon J, Foreman NK, et al.: Conventional MRI at presentation does not predict clinical response to radiation therapy in children with diffuse pontine glioma. Pediatr Radiol 39 (12): 1317-20, 2009.
- Walker DA, Liu J, Kieran M, et al.: A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Oncol 15 (4): 462-8, 2013.
- Cage TA, Samagh SP, Mueller S, et al.: Feasibility, safety, and indications for surgical biopsy of intrinsic brainstem tumors in children. Childs Nerv Syst 29 (8): 1313-9, 2013.
- Grill J, Puget S, Andreiuolo F, et al.: Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr Blood Cancer 58 (4): 489-91, 2012.
- Puget S, Beccaria K, Blauwblomme T, et al.: Biopsy in a series of 130 pediatric diffuse intrinsic Pontine gliomas. Childs Nerv Syst 31 (10): 1773-80, 2015.
- Gupta N, Goumnerova LC, Manley P, et al.: Prospective feasibility and safety assessment of surgical biopsy for patients with newly diagnosed diffuse intrinsic pontine glioma. Neuro Oncol 20 (11): 1547-1555, 2018.
- Pfaff E, El Damaty A, Balasubramanian GP, et al.: Brainstem biopsy in pediatric diffuse intrinsic pontine glioma in the era of precision medicine: the INFORM study experience. Eur J Cancer 114: 27-35, 2019.
- Ryall S, Zapotocky M, Fukuoka K, et al.: Integrated Molecular and Clinical Analysis of 1,000 Pediatric Low-Grade Gliomas. Cancer Cell 37 (4): 569-583.e5, 2020.
- Buczkowicz P, Bartels U, Bouffet E, et al.: Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol 128 (4): 573-81, 2014.
- Holzapfel J, Kandels D, Schmidt R, et al.: Favorable prognosis in pediatric brainstem low-grade glioma: Report from the German SIOP-LGG 2004 cohort. Int J Cancer 146 (12): 3385-3396, 2020.
- Warren KE: Diffuse intrinsic pontine glioma: poised for progress. Front Oncol 2: 205, 2012.
- Packer RJ, Iavarone A, Jones DTW, et al.: Implications of new understandings of gliomas in children and adults with NF1: report of a consensus conference. Neuro Oncol 22 (6): 773-784, 2020.
- D'Angelo F, Ceccarelli M, Tala, et al.: The molecular landscape of glioma in patients with Neurofibromatosis 1. Nat Med 25 (1): 176-187, 2019.
- Franz DN, Agricola K, Mays M, et al.: Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann Neurol 78 (6): 929-38, 2015.
- Mackay A, Burford A, Carvalho D, et al.: Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 32 (4): 520-537.e5, 2017.
- Bouffet E, Larouche V, Campbell BB, et al.: Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol 34 (19): 2206-11, 2016.
- Das A, Tabori U, Sambira Nahum LC, et al.: Efficacy of Nivolumab in Pediatric Cancers with High Mutation Burden and Mismatch Repair Deficiency. Clin Cancer Res 29 (23): 4770-4783, 2023.
- Jones DT, Kocialkowski S, Liu L, et al.: Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res 68 (21): 8673-7, 2008.
- Hawkins C, Walker E, Mohamed N, et al.: BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clin Cancer Res 17 (14): 4790-8, 2011.
- Mistry M, Zhukova N, Merico D, et al.: BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J Clin Oncol 33 (9): 1015-22, 2015.
- López GY, Van Ziffle J, Onodera C, et al.: The genetic landscape of gliomas arising after therapeutic radiation. Acta Neuropathol 137 (1): 139-150, 2019.
- Lassaletta A, Zapotocky M, Mistry M, et al.: Therapeutic and Prognostic Implications of BRAF V600E in Pediatric Low-Grade Gliomas. J Clin Oncol 35 (25): 2934-2941, 2017.
- Ho CY, Mobley BC, Gordish-Dressman H, et al.: A clinicopathologic study of diencephalic pediatric low-grade gliomas with BRAF V600 mutation. Acta Neuropathol 130 (4): 575-85, 2015.
- Guerreiro Stucklin AS, Ryall S, Fukuoka K, et al.: Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun 10 (1): 4343, 2019.
- Clarke M, Mackay A, Ismer B, et al.: Infant High-Grade Gliomas Comprise Multiple Subgroups Characterized by Novel Targetable Gene Fusions and Favorable Outcomes. Cancer Discov 10 (7): 942-963, 2020.
- Meredith DM, Cooley LD, Dubuc A, et al.: ROS1 Alterations as a Potential Driver of Gliomas in Infant, Pediatric, and Adult Patients. Mod Pathol 36 (11): 100294, 2023.
- Jones DT, Hutter B, Jäger N, et al.: Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet 45 (8): 927-32, 2013.
- Qaddoumi I, Orisme W, Wen J, et al.: Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol 131 (6): 833-45, 2016.
- Zhang J, Wu G, Miller CP, et al.: Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 45 (6): 602-12, 2013.
- Ramkissoon LA, Horowitz PM, Craig JM, et al.: Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci U S A 110 (20): 8188-93, 2013.
- Louis DN, Perry A, Reifenberger G, et al.: The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131 (6): 803-20, 2016.
- Bandopadhayay P, Ramkissoon LA, Jain P, et al.: MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet 48 (3): 273-82, 2016.
- D'Aronco L, Rouleau C, Gayden T, et al.: Brainstem angiocentric gliomas with MYB-QKI rearrangements. Acta Neuropathol 134 (4): 667-669, 2017.
- Chan E, Bollen AW, Sirohi D, et al.: Angiocentric glioma with MYB-QKI fusion located in the brainstem, rather than cerebral cortex. Acta Neuropathol 134 (4): 671-673, 2017.
- Sturm D, Orr BA, Toprak UH, et al.: New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs. Cell 164 (5): 1060-72, 2016.
- Lehman NL, Usubalieva A, Lin T, et al.: Genomic analysis demonstrates that histologically-defined astroblastomas are molecularly heterogeneous and that tumors with MN1 rearrangement exhibit the most favorable prognosis. Acta Neuropathol Commun 7 (1): 42, 2019.
- Wood MD, Tihan T, Perry A, et al.: Multimodal molecular analysis of astroblastoma enables reclassification of most cases into more specific molecular entities. Brain Pathol 28 (2): 192-202, 2018.
- Hirose T, Nobusawa S, Sugiyama K, et al.: Astroblastoma: a distinct tumor entity characterized by alterations of the X chromosome and MN1 rearrangement. Brain Pathol 28 (5): 684-694, 2018.
- Lucas CG, Solomon DA, Perry A: A review of recently described genetic alterations in central nervous system tumors. Hum Pathol 96: 56-66, 2020.
- Paugh BS, Qu C, Jones C, et al.: Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol 28 (18): 3061-8, 2010.
- Bax DA, Mackay A, Little SE, et al.: A distinct spectrum of copy number aberrations in pediatric high-grade gliomas. Clin Cancer Res 16 (13): 3368-77, 2010.
- Ward SJ, Karakoula K, Phipps KP, et al.: Cytogenetic analysis of paediatric astrocytoma using comparative genomic hybridisation and fluorescence in-situ hybridisation. J Neurooncol 98 (3): 305-18, 2010.
- Pollack IF, Hamilton RL, Sobol RW, et al.: IDH1 mutations are common in malignant gliomas arising in adolescents: a report from the Children's Oncology Group. Childs Nerv Syst 27 (1): 87-94, 2011.
- Sturm D, Witt H, Hovestadt V, et al.: Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22 (4): 425-37, 2012.
- Korshunov A, Ryzhova M, Hovestadt V, et al.: Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol 129 (5): 669-78, 2015.
- Castel D, Kergrohen T, Tauziède-Espariat A, et al.: Histone H3 wild-type DIPG/DMG overexpressing EZHIP extend the spectrum diffuse midline gliomas with PRC2 inhibition beyond H3-K27M mutation. Acta Neuropathol 139 (6): 1109-1113, 2020.
- Rodriguez Gutierrez D, Jones C, Varlet P, et al.: Radiological Evaluation of Newly Diagnosed Non-Brainstem Pediatric High-Grade Glioma in the HERBY Phase II Trial. Clin Cancer Res 26 (8): 1856-1865, 2020.
- Buczkowicz P, Hoeman C, Rakopoulos P, et al.: Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 46 (5): 451-6, 2014.
- Taylor KR, Mackay A, Truffaux N, et al.: Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet 46 (5): 457-61, 2014.
- Auffret L, Ajlil Y, Tauziède-Espariat A, et al.: A new subtype of diffuse midline glioma, H3 K27 and BRAF/FGFR1 co-altered: a clinico-radiological and histomolecular characterisation. Acta Neuropathol 147 (1): 2, 2023.
- Williams EA, Brastianos PK, Wakimoto H, et al.: A comprehensive genomic study of 390 H3F3A-mutant pediatric and adult diffuse high-grade gliomas, CNS WHO grade 4. Acta Neuropathol 146 (3): 515-525, 2023.
- Jain SU, Do TJ, Lund PJ, et al.: PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat Commun 10 (1): 2146, 2019.
- Hübner JM, Müller T, Papageorgiou DN, et al.: EZHIP/CXorf67 mimics K27M mutated oncohistones and functions as an intrinsic inhibitor of PRC2 function in aggressive posterior fossa ependymoma. Neuro Oncol 21 (7): 878-889, 2019.
- Chen CCL, Deshmukh S, Jessa S, et al.: Histone H3.3G34-Mutant Interneuron Progenitors Co-opt PDGFRA for Gliomagenesis. Cell 183 (6): 1617-1633.e22, 2020.
- Mackay A, Burford A, Molinari V, et al.: Molecular, Pathological, Radiological, and Immune Profiling of Non-brainstem Pediatric High-Grade Glioma from the HERBY Phase II Randomized Trial. Cancer Cell 33 (5): 829-842.e5, 2018.
- Yeo KK, Alexandrescu S, Cotter JA, et al.: Multi-institutional study of the frequency, genomic landscape, and outcome of IDH-mutant glioma in pediatrics. Neuro Oncol 25 (1): 199-210, 2023.
- Suwala AK, Stichel D, Schrimpf D, et al.: Primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA) is a distinct type with a poor prognosis. Acta Neuropathol 141 (1): 85-100, 2021.
- Reinhardt A, Stichel D, Schrimpf D, et al.: Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol 136 (2): 273-291, 2018.
- Korshunov A, Schrimpf D, Ryzhova M, et al.: H3-/IDH-wild type pediatric glioblastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol 134 (3): 507-516, 2017.
- Becker AJ: Ganglioglioma. In: Louis DN, Ohgaki H, Wiestler OD: WHO Classification of Tumours of the Central Nervous System. 4th rev.ed. IARC Press, 2016, pp 138-41.
- Blumcke I, Spreafico R, Haaker G, et al.: Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N Engl J Med 377 (17): 1648-1656, 2017.
- Pekmezci M, Villanueva-Meyer JE, Goode B, et al.: The genetic landscape of ganglioglioma. Acta Neuropathol Commun 6 (1): 47, 2018.
- Bianchi F, Tamburrini G, Massimi L, et al.: Supratentorial tumors typical of the infantile age: desmoplastic infantile ganglioglioma (DIG) and astrocytoma (DIA). A review. Childs Nerv Syst 32 (10): 1833-8, 2016.
- Trehan G, Bruge H, Vinchon M, et al.: MR imaging in the diagnosis of desmoplastic infantile tumor: retrospective study of six cases. AJNR Am J Neuroradiol 25 (6): 1028-33, 2004 Jun-Jul.
- Wang AC, Jones DTW, Abecassis IJ, et al.: Desmoplastic Infantile Ganglioglioma/Astrocytoma (DIG/DIA) Are Distinct Entities with Frequent BRAFV600 Mutations. Mol Cancer Res 16 (10): 1491-1498, 2018.
- Blessing MM, Blackburn PR, Krishnan C, et al.: Desmoplastic Infantile Ganglioglioma: A MAPK Pathway-Driven and Microglia/Macrophage-Rich Neuroepithelial Tumor. J Neuropathol Exp Neurol 78 (11): 1011-1021, 2019.
- Greer A, Foreman NK, Donson A, et al.: Desmoplastic infantile astrocytoma/ganglioglioma with rare BRAF V600D mutation. Pediatr Blood Cancer 64 (6): , 2017.
- Louis DN, Ohgaki H, Wiestler OD: WHO Classification of Tumours of the Central Nervous System. 4th rev.ed. IARC Press, 2016.
- Stone TJ, Keeley A, Virasami A, et al.: Comprehensive molecular characterisation of epilepsy-associated glioneuronal tumours. Acta Neuropathol 135 (1): 115-129, 2018.
- Rivera B, Gayden T, Carrot-Zhang J, et al.: Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol 131 (6): 847-63, 2016.
- Matsumura N, Nobusawa S, Ito J, et al.: Multiplex ligation-dependent probe amplification analysis is useful for detecting a copy number gain of the FGFR1 tyrosine kinase domain in dysembryoplastic neuroepithelial tumors. J Neurooncol 143 (1): 27-33, 2019.
- Pages M, Lacroix L, Tauziede-Espariat A, et al.: Papillary glioneuronal tumors: histological and molecular characteristics and diagnostic value of SLC44A1-PRKCA fusion. Acta Neuropathol Commun 3: 85, 2015.
- Bridge JA, Liu XQ, Sumegi J, et al.: Identification of a novel, recurrent SLC44A1-PRKCA fusion in papillary glioneuronal tumor. Brain Pathol 23 (2): 121-8, 2013.
- Hou Y, Pinheiro J, Sahm F, et al.: Papillary glioneuronal tumor (PGNT) exhibits a characteristic methylation profile and fusions involving PRKCA. Acta Neuropathol 137 (5): 837-846, 2019.
- Sievers P, Appay R, Schrimpf D, et al.: Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol 138 (3): 497-504, 2019.
- Deng MY, Sill M, Chiang J, et al.: Molecularly defined diffuse leptomeningeal glioneuronal tumor (DLGNT) comprises two subgroups with distinct clinical and genetic features. Acta Neuropathol 136 (2): 239-253, 2018.
- Chiang JCH, Harreld JH, Orr BA, et al.: Low-grade spinal glioneuronal tumors with BRAF gene fusion and 1p deletion but without leptomeningeal dissemination. Acta Neuropathol 134 (1): 159-162, 2017.
- Chiang J, Dalton J, Upadhyaya SA, et al.: Chromosome arm 1q gain is an adverse prognostic factor in localized and diffuse leptomeningeal glioneuronal tumors with BRAF gene fusion and 1p deletion. Acta Neuropathol 137 (1): 179-181, 2019.
- Sievers P, Stichel D, Schrimpf D, et al.: FGFR1:TACC1 fusion is a frequent event in molecularly defined extraventricular neurocytoma. Acta Neuropathol 136 (2): 293-302, 2018.
- Wisoff JH, Sanford RA, Heier LA, et al.: Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children's Oncology Group. Neurosurgery 68 (6): 1548-54; discussion 1554-5, 2011.
- Bandopadhayay P, Bergthold G, London WB, et al.: Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer 61 (7): 1173-9, 2014.
- Lu VM, Di L, Gernsback J, et al.: Contemporary outcomes of diffuse leptomeningeal glioneuronal tumor in pediatric patients: A case series and literature review. Clin Neurol Neurosurg 218: 107265, 2022.
- Stokland T, Liu JF, Ironside JW, et al.: A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro Oncol 12 (12): 1257-68, 2010.
- Gnekow AK, Walker DA, Kandels D, et al.: A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma - A final report. Eur J Cancer 81: 206-225, 2017.
- Chamdine O, Broniscer A, Wu S, et al.: Metastatic Low-Grade Gliomas in Children: 20 Years' Experience at St. Jude Children's Research Hospital. Pediatr Blood Cancer 63 (1): 62-70, 2016.
- Due-Tønnessen BJ, Helseth E, Scheie D, et al.: Long-term outcome after resection of benign cerebellar astrocytomas in children and young adults (0-19 years): report of 110 consecutive cases. Pediatr Neurosurg 37 (2): 71-80, 2002.
- Massimi L, Tufo T, Di Rocco C: Management of optic-hypothalamic gliomas in children: still a challenging problem. Expert Rev Anticancer Ther 7 (11): 1591-610, 2007.
- Campagna M, Opocher E, Viscardi E, et al.: Optic pathway glioma: long-term visual outcome in children without neurofibromatosis type-1. Pediatr Blood Cancer 55 (6): 1083-8, 2010.
- Hernáiz Driever P, von Hornstein S, Pietsch T, et al.: Natural history and management of low-grade glioma in NF-1 children. J Neurooncol 100 (2): 199-207, 2010.
- Falzon K, Drimtzias E, Picton S, et al.: Visual outcomes after chemotherapy for optic pathway glioma in children with and without neurofibromatosis type 1: results of the International Society of Paediatric Oncology (SIOP) Low-Grade Glioma 2004 trial UK cohort. Br J Ophthalmol 102 (10): 1367-1371, 2018.
- Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al.: Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol 36 (19): 1963-1972, 2018.
- Cohen KJ, Pollack IF, Zhou T, et al.: Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group. Neuro Oncol 13 (3): 317-23, 2011.
- Rodriguez D, Calmon R, Aliaga ES, et al.: MRI and Molecular Characterization of Pediatric High-Grade Midline Thalamic Gliomas: The HERBY Phase II Trial. Radiology 304 (1): 174-182, 2022.
- McAbee JH, Modica J, Thompson CJ, et al.: Cervicomedullary tumors in children. J Neurosurg Pediatr 16 (4): 357-66, 2015.
- Ballester LY, Wang Z, Shandilya S, et al.: Morphologic characteristics and immunohistochemical profile of diffuse intrinsic pontine gliomas. Am J Surg Pathol 37 (9): 1357-64, 2013.
- Wu G, Diaz AK, Paugh BS, et al.: The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46 (5): 444-50, 2014.
- Hoffman LM, DeWire M, Ryall S, et al.: Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol Commun 4: 1, 2016.
- Erker C, Lane A, Chaney B, et al.: Characteristics of patients ≥10 years of age with diffuse intrinsic pontine glioma: a report from the International DIPG/DMG Registry. Neuro Oncol 24 (1): 141-152, 2022.
- Broniscer A, Laningham FH, Sanders RP, et al.: Young age may predict a better outcome for children with diffuse pontine glioma. Cancer 113 (3): 566-72, 2008.
- Pascual-Castroviejo I, Pascual-Pascual SI, Viaño J, et al.: Posterior fossa tumors in children with neurofibromatosis type 1 (NF1). Childs Nerv Syst 26 (11): 1599-603, 2010.
- Albers AC, Gutmann DH: Gliomas in patients with neurofibromatosis type 1. Expert Rev Neurother 9 (4): 535-9, 2009.
Stage Information for Childhood Astrocytomas, Other Gliomas, and Glioneuronal / Neuronal Tumors
There is no recognized staging system for childhood astrocytomas, other gliomas, and glioneuronal/neuronal tumors. Unifocal disease represents by far the most common initial clinical presentation, followed by multifocal and/or diffuse disease, including leptomeningeal disease. Disease spread outside the central nervous system (CNS) is exceedingly rare.
Spread of diffuse midline glioma in the pons, noted clinically, is usually contiguous, with metastasis via the subarachnoid space. Such dissemination may occur before local progression but usually occurs simultaneously with or after primary disease progression.[1] However, subclinically, more widespread dissemination with extension to the brain stem, thalamus, cerebrum, and supratentorial leptomeninges has been noted at autopsy.[2]
References:
- Sethi R, Allen J, Donahue B, et al.: Prospective neuraxis MRI surveillance reveals a high risk of leptomeningeal dissemination in diffuse intrinsic pontine glioma. J Neurooncol 102 (1): 121-7, 2011.
- Caretti V, Bugiani M, Freret M, et al.: Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathol 128 (4): 605-7, 2014.
Treatment Option Overview for Childhood Astrocytomas, Other Gliomas, and Glioneuronal / Neuronal Tumors
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2020, childhood cancer mortality decreased by more than 50%.[1] Many of the improvements in survival in childhood cancer have been made as a result of clinical trials that have attempted to improve on the best available, accepted therapy. Clinical trials in pediatrics are designed to compare new therapy with therapy that is currently accepted as standard. This comparison may be done in a randomized study of two treatment arms or by evaluating a single new treatment and comparing the results with previously obtained results that assessed an existing therapy. Because of the relative rarity of cancer in children, all patients with brain tumors should be considered for entry into a clinical trial. Information about ongoing National Cancer Institute (NCI)–supported clinical trials is available from the NCI website.
To determine and implement optimal treatment, planning by a multidisciplinary team of cancer specialists who have experience treating childhood brain tumors is required. Irradiation of pediatric brain tumors is technically very demanding and should be carried out in centers that have experience in that area to ensure optimal results.
Long-term management of patients with brain tumors is complex and requires a multidisciplinary approach. For information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors, see Late Effects of Treatment for Childhood Cancer.
Table 3 describes the standard treatment options for childhood astrocytomas, other gliomas, and glioneuronal/neuronal tumors.
| Treatment Group | Standard Treatment Options | |
|---|---|---|
| Circumscribed astrocytic gliomas, pediatric-type diffuse low-grade gliomas, and glioneuronal/neuronal tumors: | ||
| Newly diagnosed | Observation without intervention | |
| Surgery | ||
| Adjuvant therapy: | ||
| — Observation after surgery(no adjuvant therapy) | ||
| — Chemotherapy | ||
| — Radiation therapy | ||
| — Targeted therapy | ||
| Progressive/recurrent | Second surgery | |
| Radiation therapy | ||
| Chemotherapy | ||
| Targeted therapy | ||
| Pediatric-type diffuse high-grade gliomas: | ||
| Newly diagnosed | Surgery | |
| Adjuvant therapy: | ||
| — Radiation therapy | ||
| — Chemotherapy | ||
| Targeted therapy | ||
| Immunotherapy | ||
| Recurrent | Second surgery(not considered standard treatment) | |
| Radiation therapy(not considered standard treatment) | ||
| Targeted therapy(not considered standard treatment) | ||
| Immunotherapy(not considered standard treatment) | ||
References:
- Surveillance Research Program, National Cancer Institute: SEER*Explorer: An interactive website for SEER cancer statistics. Bethesda, MD: National Cancer Institute. Available online. Last accessed March 6, 2024.
Treatment of Circumscribed Astrocytic Gliomas, Pediatric-Type Diffuse Low-Grade Gliomas, and Glioneuronal / Neuronal Tumors
To determine and implement optimal management, treatment is best guided by a multidisciplinary team of specialists experienced in treating pediatric patients with brain tumors.
For children with optic pathway gliomas, an important primary goal of treatment is preservation of visual function.[1]
Standard treatment options for newly diagnosed circumscribed astrocytic gliomas, pediatric-type diffuse low-grade gliomas, and glioneuronal/neuronal tumors include the following:
- Observation without intervention.
- Surgery.
- Adjuvant therapy.
- Observation after surgery.
- Chemotherapy.
- Radiation therapy.
- Targeted therapy.
Observation Without Intervention
Observation, without any intervention, is an option for patients with neurofibromatosis type 1 (NF1) or incidentally found, asymptomatic tumors.[2] Spontaneous regressions of optic pathway gliomas have been reported in children with and without NF1.[3,4]
Surgery
Surgical resection is a primary treatment,[5,6] and surgical feasibility depends on tumor location. For example, safe surgical resection may not be feasible in many patients with optic pathway gliomas, because even a biopsy may present risks to the patient's vision. As a result, a diagnosis of an optic pathway glioma may rely on a compatible history and imaging findings alone. This is especially true in patients with NF1.[5] For other clinical presentations of an optic pathway tumor, particularly when the tumor is more infiltrative, a biopsy may be considered for molecular characterization of the tumor.
For patients presenting with obstructive hydrocephalus, a shunt or other cerebrospinal fluid diversion procedure may also be needed.
- Cerebellum: Complete or near-complete removal can be obtained in 90% to 95% of patients with pilocytic astrocytomas located in the cerebellum.[6]
- Optic nerve: For children with isolated optic nerve lesions and progressive symptoms, complete surgical resection, while curative, generally results in blindness in the affected eye. In the absence of retained vision in the affected eye, complete surgical resection may be considered when cosmesis related to proptosis is of concern.
- Midline structures (hypothalamus, thalamus, and brain stem): Circumscribed astrocytic gliomas located in midline structures can sometimes be aggressively resected, with resultant long-term disease control.[3] Despite the increasing surgical accessibility of these tumors, such resection may result in significant neurological sequelae, especially in children younger than 2 years at diagnosis.[7][Level of evidence C1] For pediatric-type diffuse low-grade gliomas in deep-seated lesions, extensive surgical resection may not be appropriate and biopsy only should be considered.[8][Level of evidence C2]
In general, for focal brain stem gliomas, particularly those arising in the pons and medulla, maximal safe surgical resection is attempted.[9] While a greater extent of resection is associated with a higher progression-free survival (PFS), this must be balanced with the risk of new postsurgical complications. In a series of 116 patients with low-grade gliomas of the brain stem, 100 patients had some surgical intervention. Twenty-seven patients underwent a biopsy, only one of whom had new postoperative deficits. Seventy-three patients underwent a complete or partial resection, and almost 30% of this group had significant postoperative complications, including respiratory insufficiency (five patients), cerebellar mutism (three patients), and cranial nerve palsies or paresis (15 patients).[10]
- Cerebrum: Hemispheric circumscribed astrocytic gliomas are often amenable to complete surgical resection.
- Spine: Surgical resection of spinal tumors is generally attempted but it often cannot be completed. In a cohort of 128 patients with primary spinal cord low-grade gliomas, gross-total resection was achieved in a minority of the patients (24 of 128). For the entire cohort, long-term disease control was achieved in about 87% of patients, but subsequent treatment in the form of repeat resection, chemotherapy, and/or radiation therapy was frequently required. Notably, disease progression was common (51 of 128 patients), with late-progression events occurring often. Neurological sequelae and orthopedic complications were common.[11][Level of evidence C2]
After resection, immediate (within 48 hours of resection per Children's Oncology Group [COG] criteria) postoperative magnetic resonance imaging is obtained. Surveillance scans are then obtained periodically for completely resected tumors, although the value following the initial 3- to 6-month postoperative period is uncertain.[12]; [13][Level of evidence C2]
Factors related to outcome for children with low-grade gliomas treated with surgery followed by observation were identified in a COG study that included 518 evaluable patients.[6] Overall outcome for the entire group was an 8-year PFS rate of 78% and an 8-year overall survival (OS) rate of 96%. The following factors were related to prognosis:[6]
- Tumor location: Children with cerebellar and cerebral tumors showed a higher PFS rate at 8 years compared with patients with midline and chiasmatic tumors (84% ± 1.9% vs. 51% ± 5.9%, respectively).
- Histology: Approximately three-fourths of patients had pilocytic astrocytoma; PFS and OS were superior for these patients when compared with children with nonpilocytic tumors.
- Extent of resection: Patients with gross-total resection had 8-year PFS rates exceeding 90% and OS rates of 99%. By comparison, approximately one-half of patients with any degree of residual tumor (as assessed by operative report and by postoperative imaging) showed disease progression by 8 years, although OS rates exceeded 90%.[6]
A multivariate analysis examined 100 patients with confirmed diagnoses of World Health Organization (WHO) grade 2 diffuse gliomas treated in an International Society of Paediatric Oncology (SIOP) study. The extent of glioma resection had the greatest impact on event-free survival (EFS) rates. The 5-year EFS rates were 75% to 76% for patients who underwent a complete or subtotal resection. In comparison, 5-year EFS rates were 56% for patients who had a partial resection and 19% for patients who had a biopsy.[14][Level of evidence B4]
The extent of resection necessary for cure is unknown because patients with microscopic and even gross residual tumor after surgery may experience long-term PFS without postoperative therapy.[5,6]
- Age: Younger children (age <5 years) showed higher rates of tumor progression but there was no significant age effect for OS in multivariate analysis. In a retrospective review of a different series of pediatric patients, children younger than 1 year with low-grade gliomas demonstrated an inferior PFS compared with children aged 1 year and older.[15]
The long-term functional outcome of patients with cerebellar pilocytic astrocytomas is relatively favorable. Full-scale mean intelligence quotients (IQs) of patients with low-grade gliomas treated with surgery alone are close to the normative population. However, these patients may have long-term medical, psychological, and educational deficits.[16]; [17,18][Level of evidence C1]
Adjuvant Therapy
Adjuvant therapy following complete resection is generally not required unless there is a subsequent recurrence of disease. Treatment options for patients with incompletely resected tumor must be individualized and may include one or more of the following:
- Observation after surgery (no adjuvant therapy).
- Chemotherapy.
- Radiation therapy.
- Targeted therapy (for subependymal giant cell astrocytomas).
Observation after surgery
Patients whose tumors have been partially resected may be observed without further disease-directed treatment, particularly if the pace of tumor regrowth is anticipated to be very slow. Approximately 50% of patients with less-than-gross total resections have disease that does not progress in 5 to 8 years, supporting the observation strategy in selected patients.[6]
Chemotherapy
Given the long-term side effects associated with radiation therapy, chemotherapy is recommended as first-line therapy for most pediatric patients who require adjuvant therapy after surgery.
Chemotherapy may result in objective tumor shrinkage and help avoid, or at least delay, the need for radiation therapy in most patients.[19,20,21] Chemotherapy is also an option for adolescents with optic nerve pathway gliomas to delay or avoid radiation therapy.[22][Level of evidence C2] Chemotherapy has been shown to shrink tumors in children with hypothalamic gliomas and the diencephalic syndrome, resulting in weight gain in those who respond to treatment.[23]
The most widely used regimens to treat tumor progression or symptomatic nonresectable, pediatric low-grade gliomas are the following:
- Carboplatin with or without vincristine.[20,24,25]; [26][Level of evidence C2]
- Vinblastine.[27,28]
- A combination of thioguanine, procarbazine, lomustine, and vincristine (TPCV).[29]; [19][Level of evidence A1]
The COG reported the results of a randomized phase III trial (COG-A9952) that treated children younger than 10 years with low-grade chiasmatic/hypothalamic gliomas without NF1 using one of two regimens: carboplatin and vincristine (CV) or TPCV. The 5-year EFS rate was 39% (± 4%) for patients who received the CV regimen and 52% (± 5%) for patients who received the TPCV regimen. Toxicity rates between the two regimens were relatively comparable.[19] In the same study, children with NF1 were nonrandomly assigned to receive treatment with CV. The 5-year EFS rate for children with NF1 was markedly better, at 69% (± 4%), than it was for children without NF1 who received CV. In multivariate analysis, NF1 was an independent predictor of better EFS but not OS.[30] In a separate study that included 100 patients with WHO grade 2 diffuse gliomas, a subset of patients (n = 16) were treated with CV, and some patients also received etoposide. This subset of patients had a 5-year PFS rate of 38% when patients with histone H3 variants were excluded.[14][Level of evidence B4]
Other chemotherapy approaches that have been employed to treat children with progressive or symptomatic nonresectable, low-grade astrocytomas include the following:
- Multiagent, platinum-based regimens.[20,21,31]; [32][Level of evidence B4]; [33][Level of evidence C1] Reported 5-year PFS rates have ranged from approximately 35% to 60% for children who received platinum-based chemotherapy for optic pathway gliomas,[20,21] but most patients ultimately require further treatment. This is particularly true for children who initially present with hypothalamic/chiasmatic gliomas that have neuraxis dissemination.[34][Level of evidence C2]
- Temozolomide.[35,36]
Among children who received chemotherapy for optic pathway gliomas, those without NF1 had higher rates of disease progression than those with NF1, and infants had higher rates of disease progression than children older than 1 year.[20,21,28] Visual status (including acuity and field) is an important measure of outcome and response to treatment. Vision function can be impaired; it is variable even in patients with radiographic responses and is often less than optimal. More than one-third of patients successfully treated with chemotherapy have poor vision in one or both eyes, and some patients lose vision despite radiographic evidence of tumor control (response or stability). In most series, children with sporadic visual pathway gliomas have poorer visual outcomes than do children with NF1.[28]; [37,38][Level of evidence C1] Better initial visual acuity, older age, and absence of postchiasmatic involvement are associated with improved or stable vision after chemotherapy.[39,40]
Radiation therapy
Radiation therapy is usually reserved for patients with disease that does not durably respond to chemotherapy.[20,21,41,42]
For children with low-grade gliomas for whom radiation therapy is indicated, approaches that contour the radiation distribution to the tumor and avoid normal brain tissue (3-D conformal radiation therapy, intensity-modulated radiation therapy (IMRT), stereotactic radiation therapy, and proton radiation therapy [charged-particle radiation therapy]) can reduce the acute and long-term toxicities associated with these modalities.[43,44]; [45][Level of evidence C2] Radiation doses of 54 Gy in 1.8 Gy fractions are typically used.[46,47] In a prospective study of 174 patients treated with proton therapy, the 5-year actuarial rate of local control was 85% (95% confidence interval [CI], 78%–90%), the PFS rate was 84% (95% CI, 77%–89%), and the OS rate was 92% (95% CI, 85%–95%). Brain stem and spinal cord tumor locations and a dose of 54 Gy relative biological effectiveness (RBE) or less were associated with inferior local control (P < .01 for both).[48] In a separate study that included 100 patients with WHO grade 2 diffuse gliomas, a subset of patients (n = 16) were treated with radiation therapy. These patients had a 5-year PFS rate of 74% when patients with histone H3 variants were excluded.[14][Level of evidence B4]
Subsequent to radiation therapy administration, care must be taken to distinguish radiation-induced imaging changes, termed pseudoprogression or spurious progression,[49] from disease progression. The peak time to radiation therapy–induced imaging changes, often presenting as an apparent enlargement of the irradiated mass, is 4 to 6 months, but they can manifest even later.[50,51,52,53]; [54,55][Level of evidence B4]; [8,56,57][Level of evidence C2] In a report of 83 patients with low-grade astrocytomas, pseudoprogression was more common after radiation doses of higher than 50.4 Gy (RBE) (hazard ratio [HR], 2.61; P = .16). Pseudoprogression was also more common after proton radiation therapy than after photon IMRT (HR, 2.15; P = .048), presumably because of increased effects on the vasculature. Patients with pilocytic histology had lower rates of pseudoprogression than those with nonpilocytic low-grade gliomas (HR, 0.47; P = .037). There was no association with overall disease control.[49]
A report from the SIOP-LGG 2004 (NCT00276640) study and LGG-registry cohorts evaluated the following radiological criteria for pseudoprogression:[58]
- Increasing total tumor–associated T2 lesion.
- Increasing focal tumor–associated T2 lesion.
- Increasing contrast-enhancing tumor in the first 24 months after radiation therapy.
The following results were observed:
- Definite pseudoprogression was radiologically determined in 54 of 136 patients (39.7%) without differences in frequency between radiation therapy modalities: iodine-interstitial radiation therapy (22 of 48 patients) versus photon radiation therapy (24 of 54 patients) versus proton-beam radiation therapy (11 of 20 patients) (P = .780).
- Definite pseudoprogression occurred at median 6.3 months (iodine-interstitial radiation therapy, 7.2 months; photon radiation therapy, 4.4 months; proton-beam radiation therapy, 6.5 months) after radiation therapy initiation and persisted for a median of 7.2 months (iodine-interstitial radiation therapy, 8.5 months; photon radiation therapy, 7 months; proton-beam radiation therapy, 7.4 months).
- Appearance of necrosis within the focal tumor–associated T2 lesion proved to be a relevant predictor of definite pseudoprogression (P < .001).
Radiation therapy results in long-term radiographic disease control for most children with chiasmatic and posterior pathway chiasmatic gliomas. However, despite radiological control, visual outcomes are variable.
- A study from St. Jude Children's Research Hospital reported on long-term visual acuity outcomes after radiation therapy. For the worse eye, the 5-year cumulative incidence of visual acuity decline was 17.9% and improvement was 13.5%. For the better eye, the 5-year cumulative incidence of visual acuity decline was 11.5% and improvement was 10.6%. After radiation therapy, most patients had stabilization of their vision. Visual change after radiation therapy was most likely to occur within 2 years, supporting the importance of visual assessments during this period.[59]
- Another study of 38 patients (mean age, 3 years; median follow-up, 8.5 years) with optic pathway gliomas treated between 2000 and 2018 complemented the previous data on preservation of long-term visual acuity. For patients treated with early radiation therapy (either up-front or as first salvage), blindness-free survival rates were 100% at 5 and 8 years. In comparison, blindness-free survival rates were 81% at 5 years and 60% at 8 years for patients treated primarily with chemotherapy.[60]
- Other sequelae include intellectual and endocrinologic deterioration, cerebrovascular damage, late death, and possibly an increased risk of secondary tumors.[61,62,63]; [55][Level of evidence B4] A population-based study identified radiation therapy as the most significant risk factor associated with late mortality, although the patients who required radiation therapy may have reflected a higher-risk population.[63]
The management of unresectable circumscribed astrocytic gliomas, pediatric-type diffuse low-grade gliomas, glioneuronal tumors, and neuronal tumors is controversial. To identify negative prognostic features in patients treated with radiation therapy, the St. Jude Children's Research Hospital assessed 150 children (median age, 8 years; range, 1.2–20 years) who received radiation therapy and were monitored for a median of 11.4 years (range, 0.24–29.4 years). Recursive positioning analysis yielded low-risk and high-risk prognostic groups. The 10-year OS rate was 95.6% for patients in the low-risk group, versus 76.4% for patients in the high-risk group. Low-risk tumors included pilocytic astrocytoma/ganglioglioma located outside of the midbrain/thalamus, while high-risk tumors included diffuse astrocytoma or those located in the midbrain/thalamus. Within the high-risk group of patients, delayed radiation therapy (defined as after at least one line of chemotherapy) was associated with a decrement in OS.[64]
Children with NF1 may be at higher risk of radiation-associated secondary tumors and morbidity resulting from vascular changes. Radiation therapy is used as a last resort in these patients, given the heightened risk of inducing neurological toxic effects and second malignancy.[65]
Targeted therapy
The U.S. Food and Drug Administration approved the combination of trametinib (MEK inhibitor) plus dabrafenib (BRAF inhibitor) for the treatment of pediatric patients aged 1 year and older with low-grade gliomas and a BRAF V600E variant who require systemic therapy. The approval was based on a randomized clinical trial that compared the dabrafenib-plus-trametinib combination with the carboplatin-plus-vincristine combination. The median age of enrolled patients was 9.5 years, and the most common histological subtypes were ganglioglioma (about 25%) and pilocytic astrocytoma (about 30%). Patients were randomly assigned in a 2-to-1 ratio, with 73 receiving dabrafenib plus trametinib and 37 receiving carboplatin plus vincristine. Patients received dabrafenib and trametinib until loss of clinical benefit or until unacceptable toxicity, and the carboplatin-plus-vincristine combination was given as a 10-week induction course, followed by eight 6-week cycles of therapy.[66]
- The objective response rate was assessed by independent review using Response Assessment in Neuro-Oncology (RANO) 2017 response criteria for low-grade glioma that employ T2-fluid attenuated inversion recovery (FLAIR) rather than contrast enhancement.
- Patients randomly assigned to dabrafenib plus trametinib had a significantly higher objective response rate compared with patients who received carboplatin plus vincristine (47% vs. 11%). An additional 41% of patients in each treatment group had stable disease.
- Patients randomly assigned to dabrafenib plus trametinib had a significantly longer PFS compared with patients who received carboplatin plus vincristine (20.1 months vs. 7.4 months).
- Grade 3 or higher adverse events were more common in patients who received carboplatin plus vincristine compared with patients who received dabrafenib plus trametinib (94% vs. 47%).
IDH inhibitors are being studied for the treatment of patients with IDH-altered low-grade and high-grade gliomas. One agent, vorasidenib, has shown preliminary evidence of activity in delaying the time to progression when compared with placebo in newly diagnosed adults with IDH1- or IDH2-altered low-grade gliomas.[67]
For children with tuberous sclerosis (TS) and symptomatic subependymal giant cell astrocytomas (SEGAs), agents that inhibit mammalian target of rapamycin (mTOR) (e.g., everolimus and sirolimus) have been studied.
Evidence (treatment of SEGA with an mTOR inhibitor):
- Small series have shown significant reductions in the size of these tumors after administration of everolimus or sirolimus, often eliminating the need for surgery.[68]; [69][Level of evidence B4]; [70][Level of evidence C3]; [71][Level of evidence C1]
- A multicenter, phase III, placebo-controlled trial of 117 patients confirmed these earlier findings.[72][Level of evidence B3]
- Thirty-five percent of the patients in the everolimus group had at least a 50% reduction in the size of the SEGA, versus no reduction in the placebo group.
- In a study of patients who were treated with everolimus for 5 years, the following results were observed:[73]
- A reduction in the size of the mass was observed in about 50% of patients; in many cases, the reduction was sustained.
- These patients also had a reduction in seizure frequency.
Treatment Options Under Clinical Evaluation
Early-phase therapeutic trials may be available for selected patients. These trials may be available via the COG, the Pediatric Brain Tumor Consortium, or other entities. Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- ACNS1831 (NCT03871257) (A Study of the Drugs Selumetinib Versus Carboplatin/Vincristine in Patients With NF1 and Low-Grade Glioma): This phase III trial investigates the use of selumetinib compared with the standard treatment of CV for treating patients with NF1-associated low-grade gliomas, and improving vision in patients with low-grade gliomas of the optic pathway (vision nerves).
- ACNS1833 (NCT04166409) (A Study of the Drugs Selumetinib Versus Carboplatin and Vincristine in Patients With Low-Grade Glioma): This phase III trial compares the effect of selumetinib with the standard of care treatment using carboplatin and vincristine in treating patients with newly diagnosed or previously untreated low-grade glioma that does not have a BRAF V600E variant and is not associated with systemic NF1.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Nicolin G, Parkin P, Mabbott D, et al.: Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer 53 (7): 1231-7, 2009.
- Listernick R, Ferner RE, Liu GT, et al.: Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol 61 (3): 189-98, 2007.
- Albright AL: Feasibility and advisability of resections of thalamic tumors in pediatric patients. J Neurosurg 100 (5 Suppl Pediatrics): 468-72, 2004.
- Piccirilli M, Lenzi J, Delfinis C, et al.: Spontaneous regression of optic pathways gliomas in three patients with neurofibromatosis type I and critical review of the literature. Childs Nerv Syst 22 (10): 1332-7, 2006.
- Due-Tønnessen BJ, Helseth E, Scheie D, et al.: Long-term outcome after resection of benign cerebellar astrocytomas in children and young adults (0-19 years): report of 110 consecutive cases. Pediatr Neurosurg 37 (2): 71-80, 2002.
- Wisoff JH, Sanford RA, Heier LA, et al.: Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children's Oncology Group. Neurosurgery 68 (6): 1548-54; discussion 1554-5, 2011.
- Scheinemann K, Bartels U, Huang A, et al.: Survival and functional outcome of childhood spinal cord low-grade gliomas. Clinical article. J Neurosurg Pediatr 4 (3): 254-61, 2009.
- Sawamura Y, Kamada K, Kamoshima Y, et al.: Role of surgery for optic pathway/hypothalamic astrocytomas in children. Neuro Oncol 10 (5): 725-33, 2008.
- Kestle J, Townsend JJ, Brockmeyer DL, et al.: Juvenile pilocytic astrocytoma of the brainstem in children. J Neurosurg 101 (1 Suppl): 1-6, 2004.
- Holzapfel J, Kandels D, Schmidt R, et al.: Favorable prognosis in pediatric brainstem low-grade glioma: Report from the German SIOP-LGG 2004 cohort. Int J Cancer 146 (12): 3385-3396, 2020.
- Perwein T, Benesch M, Kandels D, et al.: High frequency of disease progression in pediatric spinal cord low-grade glioma (LGG): management strategies and results from the German LGG study group. Neuro Oncol 23 (7): 1148-1162, 2021.
- Sutton LN, Cnaan A, Klatt L, et al.: Postoperative surveillance imaging in children with cerebellar astrocytomas. J Neurosurg 84 (5): 721-5, 1996.
- Dorward IG, Luo J, Perry A, et al.: Postoperative imaging surveillance in pediatric pilocytic astrocytomas. J Neurosurg Pediatr 6 (4): 346-52, 2010.
- Falkenstein F, Gessi M, Kandels D, et al.: Prognostic impact of distinct genetic entities in pediatric diffuse glioma WHO-grade II-Report from the German/Swiss SIOP-LGG 2004 cohort. Int J Cancer 147 (8): 2159-2175, 2020.
- Mirow C, Pietsch T, Berkefeld S, et al.: Children <1 year show an inferior outcome when treated according to the traditional LGG treatment strategy: a report from the German multicenter trial HIT-LGG 1996 for children with low grade glioma (LGG). Pediatr Blood Cancer 61 (3): 457-63, 2014.
- Beebe DW, Ris MD, Armstrong FD, et al.: Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130). J Clin Oncol 23 (22): 5198-204, 2005.
- Turner CD, Chordas CA, Liptak CC, et al.: Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer 53 (3): 417-23, 2009.
- Daszkiewicz P, Maryniak A, Roszkowski M, et al.: Long-term functional outcome of surgical treatment of juvenile pilocytic astrocytoma of the cerebellum in children. Childs Nerv Syst 25 (7): 855-60, 2009.
- Ater JL, Zhou T, Holmes E, et al.: Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J Clin Oncol 30 (21): 2641-7, 2012.
- Gnekow AK, Falkenstein F, von Hornstein S, et al.: Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol 14 (10): 1265-84, 2012.
- Laithier V, Grill J, Le Deley MC, et al.: Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy--results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol 21 (24): 4572-8, 2003.
- Chong AL, Pole JD, Scheinemann K, et al.: Optic pathway gliomas in adolescence--time to challenge treatment choices? Neuro Oncol 15 (3): 391-400, 2013.
- Gropman AL, Packer RJ, Nicholson HS, et al.: Treatment of diencephalic syndrome with chemotherapy: growth, tumor response, and long term control. Cancer 83 (1): 166-72, 1998.
- Gururangan S, Cavazos CM, Ashley D, et al.: Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol 20 (13): 2951-8, 2002.
- Mahoney DH, Cohen ME, Friedman HS, et al.: Carboplatin is effective therapy for young children with progressive optic pathway tumors: a Pediatric Oncology Group phase II study. Neuro Oncol 2 (4): 213-20, 2000.
- Dodgshun AJ, Maixner WJ, Heath JA, et al.: Single agent carboplatin for pediatric low-grade glioma: A retrospective analysis shows equivalent efficacy to multiagent chemotherapy. Int J Cancer 138 (2): 481-8, 2016.
- Bouffet E, Jakacki R, Goldman S, et al.: Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol 30 (12): 1358-63, 2012.
- Lassaletta A, Scheinemann K, Zelcer SM, et al.: Phase II Weekly Vinblastine for Chemotherapy-Naïve Children With Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J Clin Oncol 34 (29): 3537-3543, 2016.
- Prados MD, Edwards MS, Rabbitt J, et al.: Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol 32 (3): 235-41, 1997.
- Ater JL, Xia C, Mazewski CM, et al.: Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children's Oncology Group. Cancer 122 (12): 1928-36, 2016.
- Massimino M, Spreafico F, Cefalo G, et al.: High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol 20 (20): 4209-16, 2002.
- Massimino M, Spreafico F, Riva D, et al.: A lower-dose, lower-toxicity cisplatin-etoposide regimen for childhood progressive low-grade glioma. J Neurooncol 100 (1): 65-71, 2010.
- Mora J, Perez-Jaume S, Cruz O: Treatment of childhood astrocytomas with irinotecan and cisplatin. Clin Transl Oncol 20 (4): 500-507, 2018.
- von Hornstein S, Kortmann RD, Pietsch T, et al.: Impact of chemotherapy on disseminated low-grade glioma in children and adolescents: report from the HIT-LGG 1996 trial. Pediatr Blood Cancer 56 (7): 1046-54, 2011.
- Gururangan S, Fisher MJ, Allen JC, et al.: Temozolomide in children with progressive low-grade glioma. Neuro Oncol 9 (2): 161-8, 2007.
- Khaw SL, Coleman LT, Downie PA, et al.: Temozolomide in pediatric low-grade glioma. Pediatr Blood Cancer 49 (6): 808-11, 2007.
- Moreno L, Bautista F, Ashley S, et al.: Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer 46 (12): 2253-9, 2010.
- Shofty B, Ben-Sira L, Freedman S, et al.: Visual outcome following chemotherapy for progressive optic pathway gliomas. Pediatr Blood Cancer 57 (3): 481-5, 2011.
- Falzon K, Drimtzias E, Picton S, et al.: Visual outcomes after chemotherapy for optic pathway glioma in children with and without neurofibromatosis type 1: results of the International Society of Paediatric Oncology (SIOP) Low-Grade Glioma 2004 trial UK cohort. Br J Ophthalmol 102 (10): 1367-1371, 2018.
- Rakotonjanahary J, Gravier N, Lambron J, et al.: Long-term visual acuity in patients with optic pathway glioma treated during childhood with up-front BB-SFOP chemotherapy-Analysis of a French pediatric historical cohort. PLoS One 14 (3): e0212107, 2019.
- Fisher BJ, Leighton CC, Vujovic O, et al.: Results of a policy of surveillance alone after surgical management of pediatric low grade gliomas. Int J Radiat Oncol Biol Phys 51 (3): 704-10, 2001.
- Tsang DS, Murphy ES, Merchant TE: Radiation Therapy for Optic Pathway and Hypothalamic Low-Grade Gliomas in Children. Int J Radiat Oncol Biol Phys 99 (3): 642-651, 2017.
- Greenberger BA, Pulsifer MB, Ebb DH, et al.: Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys 89 (5): 1060-8, 2014.
- Paulino AC, Mazloom A, Terashima K, et al.: Intensity-modulated radiotherapy (IMRT) in pediatric low-grade glioma. Cancer 119 (14): 2654-9, 2013.
- Müller K, Gnekow A, Falkenstein F, et al.: Radiotherapy in pediatric pilocytic astrocytomas. A subgroup analysis within the prospective multicenter study HIT-LGG 1996 by the German Society of Pediatric Oncology and Hematology (GPOH). Strahlenther Onkol 189 (8): 647-55, 2013.
- Bitterman DS, MacDonald SM, Yock TI, et al.: Revisiting the Role of Radiation Therapy for Pediatric Low-Grade Glioma. J Clin Oncol 37 (35): 3335-3339, 2019.
- Cherlow JM, Shaw DWW, Margraf LR, et al.: Conformal Radiation Therapy for Pediatric Patients with Low-Grade Glioma: Results from the Children's Oncology Group Phase 2 Study ACNS0221. Int J Radiat Oncol Biol Phys 103 (4): 861-868, 2019.
- Indelicato DJ, Rotondo RL, Uezono H, et al.: Outcomes Following Proton Therapy for Pediatric Low-Grade Glioma. Int J Radiat Oncol Biol Phys 104 (1): 149-156, 2019.
- Ludmir EB, Mahajan A, Paulino AC, et al.: Increased risk of pseudoprogression among pediatric low-grade glioma patients treated with proton versus photon radiotherapy. Neuro Oncol 21 (5): 686-695, 2019.
- Chawla S, Korones DN, Milano MT, et al.: Spurious progression in pediatric brain tumors. J Neurooncol 107 (3): 651-7, 2012.
- Marcus KJ, Goumnerova L, Billett AL, et al.: Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int J Radiat Oncol Biol Phys 61 (2): 374-9, 2005.
- Combs SE, Schulz-Ertner D, Moschos D, et al.: Fractionated stereotactic radiotherapy of optic pathway gliomas: tolerance and long-term outcome. Int J Radiat Oncol Biol Phys 62 (3): 814-9, 2005.
- Naftel RP, Pollack IF, Zuccoli G, et al.: Pseudoprogression of low-grade gliomas after radiotherapy. Pediatr Blood Cancer 62 (1): 35-9, 2015.
- Merchant TE, Kun LE, Wu S, et al.: Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol 27 (22): 3598-604, 2009.
- Merchant TE, Conklin HM, Wu S, et al.: Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol 27 (22): 3691-7, 2009.
- Kano H, Niranjan A, Kondziolka D, et al.: Stereotactic radiosurgery for pilocytic astrocytomas part 2: outcomes in pediatric patients. J Neurooncol 95 (2): 219-29, 2009.
- Hallemeier CL, Pollock BE, Schomberg PJ, et al.: Stereotactic radiosurgery for recurrent or unresectable pilocytic astrocytoma. Int J Radiat Oncol Biol Phys 83 (1): 107-12, 2012.
- Stock A, Hancken CV, Kandels D, et al.: Pseudoprogression Is Frequent After Front-Line Radiation Therapy in Pediatric Low-Grade Glioma: Results From the German Low-Grade Glioma Cohort. Int J Radiat Oncol Biol Phys 112 (5): 1190-1202, 2022.
- Acharya S, Quesada S, Coca K, et al.: Long-term visual acuity outcomes after radiation therapy for sporadic optic pathway glioma. J Neurooncol 144 (3): 603-610, 2019.
- Hanania AN, Paulino AC, Ludmir EB, et al.: Early radiotherapy preserves vision in sporadic optic pathway glioma. Cancer 127 (13): 2358-2367, 2021.
- Jenkin D, Angyalfi S, Becker L, et al.: Optic glioma in children: surveillance, resection, or irradiation? Int J Radiat Oncol Biol Phys 25 (2): 215-25, 1993.
- Khafaga Y, Hassounah M, Kandil A, et al.: Optic gliomas: a retrospective analysis of 50 cases. Int J Radiat Oncol Biol Phys 56 (3): 807-12, 2003.
- Krishnatry R, Zhukova N, Guerreiro Stucklin AS, et al.: Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: A population-based study. Cancer 122 (8): 1261-9, 2016.
- Acharya S, Liu JF, Tatevossian RG, et al.: Risk stratification in pediatric low-grade glioma and glioneuronal tumor treated with radiation therapy: an integrated clinicopathologic and molecular analysis. Neuro Oncol 22 (8): 1203-1213, 2020.
- Grill J, Couanet D, Cappelli C, et al.: Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol 45 (3): 393-6, 1999.
- Bouffet E, Hansford JR, Garrè ML, et al.: Dabrafenib plus Trametinib in Pediatric Glioma with BRAF V600 Mutations. N Engl J Med 389 (12): 1108-1120, 2023.
- Mellinghoff IK, van den Bent MJ, Blumenthal DT, et al.: Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N Engl J Med 389 (7): 589-601, 2023.
- Franz DN, Agricola KD, Tudor CA, et al.: Everolimus for tumor recurrence after surgical resection for subependymal giant cell astrocytoma associated with tuberous sclerosis complex. J Child Neurol 28 (5): 602-7, 2013.
- Krueger DA, Care MM, Holland K, et al.: Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med 363 (19): 1801-11, 2010.
- Weidman DR, Pole JD, Bouffet E, et al.: Dose-level response rates of mTor inhibition in tuberous sclerosis complex (TSC) related subependymal giant cell astrocytoma (SEGA). Pediatr Blood Cancer 62 (10): 1754-60, 2015.
- Franz DN, Leonard J, Tudor C, et al.: Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol 59 (3): 490-8, 2006.
- Franz DN, Belousova E, Sparagana S, et al.: Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 381 (9861): 125-32, 2013.
- Franz DN, Agricola K, Mays M, et al.: Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann Neurol 78 (6): 929-38, 2015.
Treatment of Progressive / Recurrent Circumscribed Astrocytic Gliomas, Pediatric-Type Diffuse Low-Grade Gliomas, and Glioneuronal / Neuronal Tumors
There is no single standard treatment option for progressive/recurrent circumscribed astrocytic gliomas, pediatric-type diffuse low-grade gliomas, glioneuronal tumors, and neuronal tumors. To determine and implement optimal management, treatment is best guided by a multidisciplinary team of specialists with experience treating pediatric patients with brain tumors.
An individual plan needs to be tailored on the basis of the following:
- Patient age.
- Tumor location.
- Pathology, including genomic findings.
- Relevant germline findings/inheritable tumor predispositions.
- Prior treatment.
Recurrent disease is usually at the primary tumor site, although multifocal or widely disseminated disease to other intracranial sites and to the spinal leptomeninges has been documented.[1,2] Most recurrences are of the same tumor entity; however, transformation into a higher grade tumor is possible and associated with the molecular profile.[3] Surveillance imaging will frequently identify asymptomatic recurrences.[4] At the time of recurrence, a complete evaluation to determine the extent of the relapse is indicated.
Tumor sample sequencing was done in pediatric (n = 48) and young adult patients (n = 6) with recurrent or refractory low-grade gliomas who were enrolled in the National Cancer Institute (NCI)–Children's Oncology Group (COG) Pediatric MATCH trial. The test revealed genomic alterations that were considered actionable for treatment on MATCH study arms in 39 of 54 tumors (72.2%).[5] Alterations in MAPK pathway genes (most commonly BRAF and NF1) were detected in 26 of 54 tumors (48.1%). FGFR1 variants (n = 11) or fusions (n = 1) were identified in 12 of 54 tumors (22.2%).
Treatment options for progressive/recurrent circumscribed astrocytic gliomas, pediatric-type diffuse low-grade gliomas, and glioneuronal/neuronal tumors include the following:
- Second surgery.
- Radiation therapy.
- Chemotherapy.
- Targeted therapy.
Second Surgery
Consideration of surgical intervention must be individualized on the basis of the following:
- Initial tumor type.
- Length of time between initial treatment and tumor recurrence/progression.
- Clinical picture.
Utility of second surgery is impacted by site of recurrence and the probability of obtaining a near-total resection/gross-total resection without significant neurological injury.[6]
Radiation Therapy
The rationale for the use of radiation therapy is essentially the same for first-line therapy or at the time of recurrence. For more information, see the Radiation therapy section. If the child has never received radiation therapy, local radiation therapy may be a treatment option, although chemotherapy in lieu of radiation should be considered, depending on the child's age and the extent and location of the tumor.[7][Level of evidence C1]; [8][Level of evidence C2]
For children with low-grade gliomas for whom radiation therapy is indicated, conformal radiation therapy (including proton-beam therapy) approaches appear effective and offer the potential for reducing the acute and long-term toxicities associated with this modality.[9,10,11,12]
Chemotherapy
If there is recurrence or progression at an unresectable site, chemotherapy should be considered.
Chemotherapy may result in relatively long-term disease control.[13,14] The choice of regimen depends on the type of and response to prior chemotherapy. Numerous options can be considered, most commonly including carboplatin with or without vincristine (CV); thioguanine, procarbazine, lomustine, and vincristine (TPCV); or vinblastine alone; temozolomide alone; temozolomide in combination with carboplatin and vincristine; irinotecan and bevacizumab; or lenalidomide.[13,14,15,16,17] When a therapeutically actionable molecular alteration is identified in the tumor, molecular targeted therapy is increasingly being used as second-line therapy.
Targeted Therapy
mTOR inhibitors
For children with tuberous sclerosis (TS) and symptomatic subependymal giant cell astrocytomas (SEGAs) or low-grade gliomas,[18] mammalian target of rapamycin (mTOR) inhibitors (e.g., everolimus and sirolimus) have been studied.
Evidence (mTOR inhibitors):
- Small series have shown significant reductions in the size of these tumors after administration of everolimus or sirolimus, often eliminating the need for surgery.[19]; [20][Level of evidence B4]; [21][Level of evidence C3]; [22][Level of evidence C1]
- A multicenter, phase III, placebo-controlled trial of 117 patients confirmed these earlier findings.[23][Level of evidence B3]
- Thirty-five percent of the patients in the everolimus group had at least a 50% reduction in the size of the SEGA, versus no reduction in the placebo group.
- In a study of patients who were treated with everolimus for 5 years, the following results were observed:[24]
- A reduction in the size of the mass was observed in about 50% of patients; in many cases, the reduction was sustained.
- These patients also had a reduction in seizure frequency.
- In a series of 23 patients with recurrent low-grade gliomas who were treated with everolimus, the following was observed:[25]
- Everolimus demonstrated modest activity, with a 2-year progression-free survival (PFS) rate of 39% and an overall survival rate of 93%.
- A companion study completed by the Neurofibromatosis Clinical Trials Consortium evaluated 23 children with neurofibromatosis type 1 (NF1) and progressive low-grade gliomas who were treated with everolimus.[26]
- Of the 22 evaluable patients, 15 demonstrated either a partial response or tumor stabilization, 10 of whom remained free of progression for a median follow-up of 33 months.
VEGF inhibitors
Antitumor activity has also been observed for bevacizumab given in combination with irinotecan, which, in some cases, also results in clinical or visual improvement.[27]
Evidence (targeted therapy [bevacizumab]):
- In a phase II study of bevacizumab plus irinotecan for children with recurrent low-grade gliomas, the following results were observed:[28]
- Sustained partial responses were observed in only two patients (5.7%).
- The 6-month PFS rate was 85.4% (standard error [SE] ± 5.96%).
- The 2-year PFS rate was 47.8% (SE ± 9.27%).
- A pilot study of 14 patients with recurrent low-grade gliomas also evaluated bevacizumab-based therapies and observed the following:[29][Level of evidence C2]; [30][Level of evidence C3]
- Objective responses were seen in 12 patients (86%).
- No patients progressed on therapy (median treatment duration, 12 months), but 13 of 14 progressed after stopping bevacizumab at a median of 5 months.
- A retrospective pooled analysis included 88 children with low-grade gliomas who received bevacizumab-based treatment along with additional therapy.[31]
- A partial response was observed in 40% of patients, and stable disease was seen in 49% of patients.
- Sixty-five percent of the patients progressed at a median of 8 months after discontinuation of bevacizumab-based treatment. The radiographic PFS rate was 29% at 3 years.
- Stability in visual function was seen in 49% of patients, and visual function improved in 29% of patients. Despite radiographic progression in many patients, the 3-year visual-PFS rate was 53%.
- Bevacizumab has also been employed for children with low-grade gliomas and symptomatic radiation-induced tumor enlargement.[32,33]
- Treatment with bevacizumab produced imaging improvement (five of five patients) and allowed weaning off steroids (four of four patients).
BRAF and MEK inhibitors
With the identification of BRAF variants driving a significant proportion of low-grade gliomas, inhibition of various elements of this molecular pathway (e.g., MEK and BRAF) are actively being tested in ongoing clinical trials, with early reports suggesting substantial activity. While first-generation BRAF inhibitors like vemurafenib and dabrafenib are active against tumors with BRAF V600E variants, they are contraindicated for tumors with BRAF gene fusions because of the potential for paradoxical activation of the MAPK pathway.[34,35] As described below, the U.S. Food and Drug Administration (FDA) approved the dabrafenib-plus-trametinib combination for use in pediatric patients aged 1 year and older with relapsed or refractory low-grade gliomas with BRAF V600E variants.
- For patients whose tumors have BRAF V600E variants, the focus of clinical research efforts is on the evaluation of BRAF inhibitors in combination with MEK inhibitors. Such combinations are approved for the treatment of adult cancers with BRAF V600E variants and are more effective than either BRAF inhibitors or MEK inhibitors used as single agents.[36]
- Results on the use of the BRAF V600E inhibitor dabrafenib demonstrated a 44% overall response rate (1 complete response and 13 partial responses) by central review in children with BRAF V600 variants and relapsed or refractory low-grade gliomas. The median duration of response was 26 months. The disease control rate (complete response plus partial response plus stable disease) was 78%. The therapy was well tolerated, although 91% of patients experienced side effects such as fatigue (34%), rash (31%), and pyrexia (28%). Nine of 32 patients had grade 3 to grade 4 toxicities, 10 patients required dose modifications, and 2 patients discontinued treatment, including 1 child who had disseminated intravascular coagulation with hypertension. In this pediatric study, no cases of squamous cell carcinoma of the skin or keratoacanthoma were encountered.[37]
- A phase I/II study of trametinib as a single agent for patients with BRAF V600E variants and low-grade gliomas enrolled 13 pediatric patients. The objective response rate for these 13 patients was assessed by independent review using Response Assessment in Neuro-Oncology (RANO) 2017 response criteria for low-grade gliomas that employ T2-fluid attenuated inversion recovery (FLAIR) rather than contrast enhancement.[38]
- Two of 13 patients (15%) achieved partial responses, and 6 patients (46%) had stable disease.
- The 24-month PFS rate was 50%.
- A phase I/II study that evaluated the combination of dabrafenib and trametinib enrolled 34 patients with BRAF V600E variants and low-grade gliomas and 2 patients with BRAF V600E variants and high-grade gliomas. The objective response rate for these 36 patients was assessed by independent review using RANO 2017 response criteria for low-grade glioma that employ T2-FLAIR rather than contrast enhancement.[38]
- Nine of 36 patients (25%) achieved partial responses, and 23 patients (64%) had stable disease.
- The 24-month PFS rate was 80%.
- The most common treatment-related adverse events in the dabrafenib-plus-trametinib group were pyrexia (50%) and dry skin (42%). Adverse events leading to discontinuation of therapy occurred in 22% of patients, a lower rate than observed for patients who received single-agent trametinib (54%).
- The FDA approved the trametinib-plus-dabrafenib combination for adult and pediatric patients aged 1 year and older with unresectable or metastatic solid tumors with BRAF V600E variants who have progressed following prior treatment and have no satisfactory alternative treatment options. This indication includes pediatric patients aged 1 year and older with BRAF V600E variants and low-grade gliomas.
- The MEK inhibitor selumetinib has been studied in a phase I/II clinical trial for children with low-grade gliomas (PBTC-029 [NCT01089101]).
- The phase I component of the PBTC-029 trial showed the following results:[39]
- Selumetinib was tolerated at a daily dose of 25 mg/m2.
- The most common adverse events leading to patient discontinuation of treatment were rash, paronychia, and asymptomatic creatine phosphokinase (CPK) elevation.
- Stratum 1 of the phase II component of this trial was for patients with BRAF genomic alterations.[40]
- Nine of 25 patients (36%) achieved a partial response, with responses occurring for both BRAF V600E patients and for patients with BRAF gene fusions.
- The 2-year PFS rate was 70% for stratum 1 patients.
- Stratum 3 of the phase II component of this trial was for patients with NF1-associated low-grade gliomas.[40]
- The 2-year event-free survival rate for this group was 96%.
- 10 of 25 patients (40%) achieved partial responses.
- Stratum 4 of the phase II component of this trial was for patients with recurrent optic pathway and hypothalamic low-grade gliomas.[41]
- Six of 25 patients (24%) had a partial response, and an additional 14 of 25 patients (56%) had stable disease.
- The 2-year PFS rate was 78%.
- Of the 19 patients evaluable for visual acuity, 4 had improvements in visual acuity, with an additional 13 having stable findings.
The most common toxicities across all strata were grade 1 and grade 2 CPK elevation, diarrhea, hypoalbuminemia, elevated aspartate aminotransferase (AST), and rash. Rare grade 3 and grade 4 toxicities included elevated CPK, rash, neutropenia, emesis, and paronychia.
- The phase I component of the PBTC-029 trial showed the following results:[39]
Treatment Options Under Clinical Evaluation
Early-phase therapeutic trials may be available for selected patients. These trials may be available via the COG, the Pediatric Brain Tumor Consortium, or other entities. Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified in a patient's tumor (refractory or recurrent). Children and adolescents aged 1 to 21 years are eligible for the trial.
Patients with tumors that have molecular variants addressed by open treatment arms in the trial may be enrolled in treatment on Pediatric MATCH. Additional information can be obtained on the NCI website and ClinicalTrials.gov website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Perilongo G, Carollo C, Salviati L, et al.: Diencephalic syndrome and disseminated juvenile pilocytic astrocytomas of the hypothalamic-optic chiasm region. Cancer 80 (1): 142-6, 1997.
- Leibel SA, Sheline GE, Wara WM, et al.: The role of radiation therapy in the treatment of astrocytomas. Cancer 35 (6): 1551-7, 1975.
- Ryall S, Zapotocky M, Fukuoka K, et al.: Integrated Molecular and Clinical Analysis of 1,000 Pediatric Low-Grade Gliomas. Cancer Cell 37 (4): 569-583.e5, 2020.
- Udaka YT, Yeh-Nayre LA, Amene CS, et al.: Recurrent pediatric central nervous system low-grade gliomas: the role of surveillance neuroimaging in asymptomatic children. J Neurosurg Pediatr 11 (2): 119-26, 2013.
- Parsons DW, Janeway KA, Patton DR, et al.: Actionable Tumor Alterations and Treatment Protocol Enrollment of Pediatric and Young Adult Patients With Refractory Cancers in the National Cancer Institute-Children's Oncology Group Pediatric MATCH Trial. J Clin Oncol 40 (20): 2224-2234, 2022.
- Bowers DC, Krause TP, Aronson LJ, et al.: Second surgery for recurrent pilocytic astrocytoma in children. Pediatr Neurosurg 34 (5): 229-34, 2001.
- Scheinemann K, Bartels U, Tsangaris E, et al.: Feasibility and efficacy of repeated chemotherapy for progressive pediatric low-grade gliomas. Pediatr Blood Cancer 57 (1): 84-8, 2011.
- de Haas V, Grill J, Raquin MA, et al.: Relapses of optic pathway tumors after first-line chemotherapy. Pediatr Blood Cancer 52 (5): 575-80, 2009.
- Merchant TE, Conklin HM, Wu S, et al.: Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol 27 (22): 3691-7, 2009.
- Marcus KJ, Goumnerova L, Billett AL, et al.: Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int J Radiat Oncol Biol Phys 61 (2): 374-9, 2005.
- Bitterman DS, MacDonald SM, Yock TI, et al.: Revisiting the Role of Radiation Therapy for Pediatric Low-Grade Glioma. J Clin Oncol 37 (35): 3335-3339, 2019.
- Cherlow JM, Shaw DWW, Margraf LR, et al.: Conformal Radiation Therapy for Pediatric Patients with Low-Grade Glioma: Results from the Children's Oncology Group Phase 2 Study ACNS0221. Int J Radiat Oncol Biol Phys 103 (4): 861-868, 2019.
- Packer RJ, Lange B, Ater J, et al.: Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol 11 (5): 850-6, 1993.
- Gnekow AK, Falkenstein F, von Hornstein S, et al.: Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol 14 (10): 1265-84, 2012.
- Lassaletta A, Scheinemann K, Zelcer SM, et al.: Phase II Weekly Vinblastine for Chemotherapy-Naïve Children With Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J Clin Oncol 34 (29): 3537-3543, 2016.
- de Marcellus C, Tauziède-Espariat A, Cuinet A, et al.: The role of irinotecan-bevacizumab as rescue regimen in children with low-grade gliomas: a retrospective nationwide study in 72 patients. J Neurooncol 157 (2): 355-364, 2022.
- Warren KE, Vezina G, Krailo M, et al.: Phase II Randomized Trial of Lenalidomide in Children With Pilocytic Astrocytomas and Optic Pathway Gliomas: A Report From the Children's Oncology Group. J Clin Oncol 41 (18): 3374-3383, 2023.
- Haas-Kogan DA, Aboian MS, Minturn JE, et al.: Everolimus for Children With Recurrent or Progressive Low-Grade Glioma: Results From the Phase II PNOC001 Trial. J Clin Oncol 42 (4): 441-451, 2024.
- Franz DN, Agricola KD, Tudor CA, et al.: Everolimus for tumor recurrence after surgical resection for subependymal giant cell astrocytoma associated with tuberous sclerosis complex. J Child Neurol 28 (5): 602-7, 2013.
- Krueger DA, Care MM, Holland K, et al.: Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med 363 (19): 1801-11, 2010.
- Weidman DR, Pole JD, Bouffet E, et al.: Dose-level response rates of mTor inhibition in tuberous sclerosis complex (TSC) related subependymal giant cell astrocytoma (SEGA). Pediatr Blood Cancer 62 (10): 1754-60, 2015.
- Franz DN, Leonard J, Tudor C, et al.: Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol 59 (3): 490-8, 2006.
- Franz DN, Belousova E, Sparagana S, et al.: Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 381 (9861): 125-32, 2013.
- Franz DN, Agricola K, Mays M, et al.: Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann Neurol 78 (6): 929-38, 2015.
- Wright KD, Yao X, London WB, et al.: A POETIC Phase II study of continuous oral everolimus in recurrent, radiographically progressive pediatric low-grade glioma. Pediatr Blood Cancer 68 (2): e28787, 2021.
- Ullrich NJ, Prabhu SP, Reddy AT, et al.: A phase II study of continuous oral mTOR inhibitor everolimus for recurrent, radiographic-progressive neurofibromatosis type 1-associated pediatric low-grade glioma: a Neurofibromatosis Clinical Trials Consortium study. Neuro Oncol 22 (10): 1527-1535, 2020.
- Avery RA, Hwang EI, Jakacki RI, et al.: Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol 132 (1): 111-4, 2014.
- Gururangan S, Fangusaro J, Poussaint TY, et al.: Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas--a Pediatric Brain Tumor Consortium study. Neuro Oncol 16 (2): 310-7, 2014.
- Hwang EI, Jakacki RI, Fisher MJ, et al.: Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr Blood Cancer 60 (5): 776-82, 2013.
- Packer RJ, Jakacki R, Horn M, et al.: Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer 52 (7): 791-5, 2009.
- Green K, Panagopoulou P, D'Arco F, et al.: A nationwide evaluation of bevacizumab-based treatments in pediatric low-grade glioma in the UK: Safety, efficacy, visual morbidity, and outcomes. Neuro Oncol 25 (4): 774-785, 2023.
- Foster KA, Ares WJ, Pollack IF, et al.: Bevacizumab for symptomatic radiation-induced tumor enlargement in pediatric low grade gliomas. Pediatr Blood Cancer 62 (2): 240-245, 2015.
- Zhukova N, Rajagopal R, Lam A, et al.: Use of bevacizumab as a single agent or in adjunct with traditional chemotherapy regimens in children with unresectable or progressive low-grade glioma. Cancer Med 8 (1): 40-50, 2019.
- Sievert AJ, Lang SS, Boucher KL, et al.: Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci U S A 110 (15): 5957-62, 2013.
- Karajannis MA, Legault G, Fisher MJ, et al.: Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol 16 (10): 1408-16, 2014.
- Odogwu L, Mathieu L, Blumenthal G, et al.: FDA Approval Summary: Dabrafenib and Trametinib for the Treatment of Metastatic Non-Small Cell Lung Cancers Harboring BRAF V600E Mutations. Oncologist 23 (6): 740-745, 2018.
- Hargrave DR, Bouffet E, Tabori U, et al.: Efficacy and Safety of Dabrafenib in Pediatric Patients with BRAF V600 Mutation-Positive Relapsed or Refractory Low-Grade Glioma: Results from a Phase I/IIa Study. Clin Cancer Res 25 (24): 7303-7311, 2019.
- Bouffet E, Geoerger B, Moertel C, et al.: Efficacy and Safety of Trametinib Monotherapy or in Combination With Dabrafenib in Pediatric BRAF V600-Mutant Low-Grade Glioma. J Clin Oncol 41 (3): 664-674, 2023.
- Banerjee A, Jakacki RI, Onar-Thomas A, et al.: A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol 19 (8): 1135-1144, 2017.
- Fangusaro J, Onar-Thomas A, Young Poussaint T, et al.: Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol 20 (7): 1011-1022, 2019.
- Fangusaro J, Onar-Thomas A, Poussaint TY, et al.: A phase II trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: a Pediatric Brain Tumor Consortium study. Neuro Oncol 23 (10): 1777-1788, 2021.
Treatment of Pediatric-Type Diffuse High-Grade Gliomas
To determine and implement optimal management, treatment is best guided by a multidisciplinary team of specialists experienced in treating pediatric patients with brain tumors.
The outcome for pediatric patients with the most common types of high-grade glioma (i.e., diffuse midline glioma, H3 K27-altered and diffuse pediatric-type high-grade glioma, H3-wild type and IDH-wild type) remains dismal.[1] In contrast, the prognosis for children with infant-type hemispheric glioma is relatively favorable.[2,3]
Maximal safe surgical resection can be considered standard of care for all patients with pediatric-type diffuse high-grade glioma.[4]
Standard adjuvant therapy for children with diffuse pediatric-type high-grade glioma, H3-wild type and IDH-wild type, includes radiation therapy and alkylator chemotherapy.[5,6,7]
For children with diffuse midline glioma, H3 K27-altered (the most common subtype), including those with diffuse intrinsic pontine glioma (DIPG), adjuvant radiation therapy alone can be considered standard of care given the apparent lack of benefit of chemotherapy.[8,9]
Standard treatment options for newly diagnosed pediatric-type diffuse high-grade gliomas include the following:
- Surgery.
- Adjuvant therapy.
- Radiation therapy.
- Chemotherapy.
- Targeted therapy.
- Immunotherapy.
Surgery
The extent of tumor resection at initial diagnosis is positively associated with survival. Therefore, maximal safe resection is recommended for children with nonpontine tumors.[4,10,11]
For children with diffuse midline glioma in the pons (DIPG), histological confirmation is increasingly obtained for both entry into research studies and molecular characterization of the tumor.[12] New approaches with stereotactic needle biopsy may make biopsy safer.[13,14,15,16] Given the technical challenges of pontine biopsies, the procedure is best undertaken by an experienced pediatric neurosurgeon to minimize the risk of irreversible neurological complications.[13,14,15,16,17] Biopsy is recommended for pontine tumors when the diagnosis is uncertain based on imaging findings.
Adjuvant Therapy
Radiation therapy
For patients with diffuse midline glioma, H3 K27-altered and diffuse pediatric-type high-grade glioma, H3-wild type and IDH-wild type, focal radiation therapy is routinely administered to a field that widely encompasses the entire tumor. The radiation therapy dose to the tumor bed is usually at least 54 Gy. Despite such therapy, the prognosis is dismal. Similarly poor survival is seen in children with spinal cord primary tumors and children with thalamic high-grade gliomas (i.e., diffuse midline gliomas, H3 K27M-altered tumors) treated with radiation therapy.[18,19]; [20,21][Level of evidence C1]
Standard treatment for children with diffuse midline gliomas centered in the pons is radiation therapy to the involved site. The conventional dose of radiation ranges between 54 Gy and 60 Gy, given locally to the primary tumor site in single daily fractions. Such treatment will result in transient benefit for most patients, but more than 90% of patients will die within 18 months of diagnosis.[22]
Radiation-induced changes may occur a few months after the completion of radiation therapy and may mimic tumor progression. When considering the efficacy of additional treatment, care needs to be taken to separate radiation-induced change from progressive disease.[23]
Research studies that evaluated the efficacy of hyperfractionated and hypofractionated radiation therapy and radiosensitizers have not demonstrated improved outcomes using these radiation techniques.
- Hyperfractionated (twice daily) radiation therapy. Studies using doses as high as 78 Gy have been completed. Evidence demonstrates that these increased radiation therapy doses do not improve the duration or rate of survival for patients with DIPGs, whether given alone [24,25] or in combination with chemotherapy, and they were associated with increased toxicity at the highest dose levels.[26]
- Hypofractionated radiation therapy. This technique results in survival rates comparable with conventional fractionated radiation therapy techniques, possibly with less treatment burden.[27]; [28][Level of evidence A1]; [22,29][Level of evidence B4] One randomized study compared three radiation therapy fractions (39 Gy in 13 fractions; 45 Gy in 15 fractions; and 54 Gy in 30 fractions). The study concluded that the higher hypofractionated regimen was inferior, possibly due to increased toxicity.[30]
- Radiosensitizers. Studies evaluating the efficacy of various radiosensitizers as a means for enhancing the therapeutic effect of radiation therapy have been completed but have failed to show any significant improvement in outcome.[25,26,31,32,33,34]
Chemotherapy
For patients with diffuse pediatric-type high-grade glioma, H3-wild type and IDH-wild type, the benefit from radiation therapy with adjuvant chemotherapy compared with radiation therapy alone has not been formally proven in a randomized prospective trial. However, the aggregate data from numerous nonrandomized prospective clinical trials for children with high-grade gliomas suggest a benefit from alkylating chemotherapy, similar to adults with primary glioblastoma. Therefore, adjuvant therapy with a combination of radiation therapy and alkylating chemotherapy can be considered standard of care. Commonly used chemotherapy regimens include temozolomide alone or in combination with lomustine.[5,6]
Prospective, randomized clinical trials in adults with primary glioblastoma have established MGMT promoter hypermethylation as an independent prognostic biomarker regardless of therapy, as well as a predictive biomarker for benefit from temozolomide.[35,36] However, in children with diffuse pediatric-type high-grade glioma, H3-wild type and IDH-wild type, MGMT promoter methylation status is not prognostic,[8,37] and its predictive value for benefit from alkylator chemotherapy is unknown given the lack of applicable randomized data.
In a prospective randomized trial, the use of adjuvant bevacizumab after radiation therapy did not prolong overall survival (OS) or progression-free survival (PFS) in pediatric patients with newly diagnosed high-grade gliomas.[7]
No chemotherapy (including neoadjuvant, concurrent, postradiation chemotherapy) or immunotherapy strategy, when added to radiation therapy, has led to long-term survival for children with DIPGs.[38,39,40]; [41][Level of evidence B4] This includes therapy using high-dose, marrow-ablative chemotherapy with autologous hematopoietic stem cell rescue, which has been shown to be ineffective in extending survival.[42] However, similar to the treatment of other brain tumors, radiation therapy is generally omitted for infants with DIPGs, and chemotherapy-only approaches are used. Published data supporting the utility of this approach are lacking.
Children with infant-type hemispheric gliomas have been categorized into three groups.[43] Group 1 tumors include high-grade gliomas that are hemispheric and receptor tyrosine kinase (RTK) driven, including ALK, NTRK, ROS1, and MET gene fusions. Previously, infants with such tumors were treated with adjuvant multiagent chemotherapy instead of radiation therapy, with relatively favorable outcomes.[9,44]
Targeted Therapy
Therapeutically targetable somatic BRAF V600E variants are present in a small subset of patients with pediatric-type diffuse high-grade gliomas. Data from a nonrandomized retrospective study suggest that up-front inclusion of BRAF and/or MEK inhibitor therapy in place of chemotherapy may result in improved survival.[45][Level of evidence C2]
There is evidence that infants with group 1 hemispheric high-grade gliomas that have specific RTK-driven gene fusions are responsive to RTK-targeted therapeutics.[43,46] A subset analysis included 33 patients with NTRK fusion–positive central nervous system tumors who were treated with larotrectinib (included in two larger trials that enrolled children and adults with solid tumors and NTRK fusions).[47] The objective response rate was 30%, and 82% of patients with measurable disease had tumor shrinkage. The 12-month duration of response rate was 75%, the PFS rate was 56%, and the OS rate was 85%.[47] The role of RTK inhibitors in the up-front treatment of infants with pediatric-type high-grade glioma remains under study.
Immunotherapy
Children with inheritable biallelic mismatch repair deficiency have a very high mutational burden and neoantigen expression. These patients are at risk of developing a variety of cancers, including hematologic malignancies, gastrointestinal cancers, and high-grade gliomas. The high variant and neoantigen load have been associated with responsiveness to immune checkpoint inhibition. Early case reports have demonstrated clinical imaging responses in children who are treated with an anti-programmed death-1 inhibitor.[48]
Treatment Options Under Clinical Evaluation
Therapeutic clinical trials may be available for selected patients. These trials may be available via the Children's Oncology Group (COG), the Pediatric Brain Tumor Consortium, or other entities. Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ACNS1723 (NCT03919071) (Dabrafenib Combined With Trametinib After Radiation Therapy in Treating Patients With Newly-Diagnosed High-Grade Glioma): This phase II trial investigates the use of the combination of dabrafenib and trametinib after radiation therapy in children and young adults with high-grade gliomas who have a BRAF V600 variant.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Mackay A, Burford A, Carvalho D, et al.: Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 32 (4): 520-537.e5, 2017.
- Clarke M, Mackay A, Ismer B, et al.: Infant High-Grade Gliomas Comprise Multiple Subgroups Characterized by Novel Targetable Gene Fusions and Favorable Outcomes. Cancer Discov 10 (7): 942-963, 2020.
- Guerreiro Stucklin AS, Ryall S, Fukuoka K, et al.: Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun 10 (1): 4343, 2019.
- Hatoum R, Chen JS, Lavergne P, et al.: Extent of Tumor Resection and Survival in Pediatric Patients With High-Grade Gliomas: A Systematic Review and Meta-analysis. JAMA Netw Open 5 (8): e2226551, 2022.
- Cohen KJ, Pollack IF, Zhou T, et al.: Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group. Neuro Oncol 13 (3): 317-23, 2011.
- Jakacki RI, Cohen KJ, Buxton A, et al.: Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children's Oncology Group ACNS0423 study. Neuro Oncol 18 (10): 1442-50, 2016.
- Grill J, Massimino M, Bouffet E, et al.: Phase II, Open-Label, Randomized, Multicenter Trial (HERBY) of Bevacizumab in Pediatric Patients With Newly Diagnosed High-Grade Glioma. J Clin Oncol 36 (10): 951-958, 2018.
- Korshunov A, Ryzhova M, Hovestadt V, et al.: Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol 129 (5): 669-78, 2015.
- Macy ME, Birks DK, Barton VN, et al.: Clinical and molecular characteristics of congenital glioblastoma. Neuro Oncol 14 (7): 931-41, 2012.
- Wisoff JH, Boyett JM, Berger MS, et al.: Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children's Cancer Group trial no. CCG-945. J Neurosurg 89 (1): 52-9, 1998.
- Yang T, Temkin N, Barber J, et al.: Gross total resection correlates with long-term survival in pediatric patients with glioblastoma. World Neurosurg 79 (3-4): 537-44, 2013 Mar-Apr.
- Walker DA, Liu J, Kieran M, et al.: A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Oncol 15 (4): 462-8, 2013.
- Cage TA, Samagh SP, Mueller S, et al.: Feasibility, safety, and indications for surgical biopsy of intrinsic brainstem tumors in children. Childs Nerv Syst 29 (8): 1313-9, 2013.
- Grill J, Puget S, Andreiuolo F, et al.: Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr Blood Cancer 58 (4): 489-91, 2012.
- Puget S, Beccaria K, Blauwblomme T, et al.: Biopsy in a series of 130 pediatric diffuse intrinsic Pontine gliomas. Childs Nerv Syst 31 (10): 1773-80, 2015.
- Gupta N, Goumnerova LC, Manley P, et al.: Prospective feasibility and safety assessment of surgical biopsy for patients with newly diagnosed diffuse intrinsic pontine glioma. Neuro Oncol 20 (11): 1547-1555, 2018.
- Pfaff E, El Damaty A, Balasubramanian GP, et al.: Brainstem biopsy in pediatric diffuse intrinsic pontine glioma in the era of precision medicine: the INFORM study experience. Eur J Cancer 114: 27-35, 2019.
- Kramm CM, Butenhoff S, Rausche U, et al.: Thalamic high-grade gliomas in children: a distinct clinical subset? Neuro Oncol 13 (6): 680-9, 2011.
- Tendulkar RD, Pai Panandiker AS, Wu S, et al.: Irradiation of pediatric high-grade spinal cord tumors. Int J Radiat Oncol Biol Phys 78 (5): 1451-6, 2010.
- Wolff B, Ng A, Roth D, et al.: Pediatric high grade glioma of the spinal cord: results of the HIT-GBM database. J Neurooncol 107 (1): 139-46, 2012.
- Ononiwu C, Mehta V, Bettegowda C, et al.: Pediatric spinal glioblastoma multiforme: current treatment strategies and possible predictors of survival. Childs Nerv Syst 28 (5): 715-20, 2012.
- Janssens GO, Jansen MH, Lauwers SJ, et al.: Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. Int J Radiat Oncol Biol Phys 85 (2): 315-20, 2013.
- Liu AK, Macy ME, Foreman NK: Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys 75 (4): 1148-54, 2009.
- Freeman CR, Krischer JP, Sanford RA, et al.: Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys 27 (2): 197-206, 1993.
- Mandell LR, Kadota R, Freeman C, et al.: There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys 43 (5): 959-64, 1999.
- Allen J, Siffert J, Donahue B, et al.: A phase I/II study of carboplatin combined with hyperfractionated radiotherapy for brainstem gliomas. Cancer 86 (6): 1064-9, 1999.
- Izzuddeen Y, Gupta S, Haresh KP, et al.: Hypofractionated radiotherapy with temozolomide in diffuse intrinsic pontine gliomas: a randomized controlled trial. J Neurooncol 146 (1): 91-95, 2020.
- Zaghloul MS, Eldebawy E, Ahmed S, et al.: Hypofractionated conformal radiotherapy for pediatric diffuse intrinsic pontine glioma (DIPG): a randomized controlled trial. Radiother Oncol 111 (1): 35-40, 2014.
- Negretti L, Bouchireb K, Levy-Piedbois C, et al.: Hypofractionated radiotherapy in the treatment of diffuse intrinsic pontine glioma in children: a single institution's experience. J Neurooncol 104 (3): 773-7, 2011.
- Zaghloul MS, Nasr A, Tolba M, et al.: Hypofractionated Radiation Therapy For Diffuse Intrinsic Pontine Glioma: A Noninferiority Randomized Study Including 253 Children. Int J Radiat Oncol Biol Phys 113 (2): 360-368, 2022.
- Freeman CR, Kepner J, Kun LE, et al.: A detrimental effect of a combined chemotherapy-radiotherapy approach in children with diffuse intrinsic brain stem gliomas? Int J Radiat Oncol Biol Phys 47 (3): 561-4, 2000.
- Broniscer A, Leite CC, Lanchote VL, et al.: Radiation therapy and high-dose tamoxifen in the treatment of patients with diffuse brainstem gliomas: results of a Brazilian cooperative study. Brainstem Glioma Cooperative Group. J Clin Oncol 18 (6): 1246-53, 2000.
- Doz F, Neuenschwander S, Bouffet E, et al.: Carboplatin before and during radiation therapy for the treatment of malignant brain stem tumours: a study by the Société Française d'Oncologie Pédiatrique. Eur J Cancer 38 (6): 815-9, 2002.
- Bradley KA, Zhou T, McNall-Knapp RY, et al.: Motexafin-gadolinium and involved field radiation therapy for intrinsic pontine glioma of childhood: a children's oncology group phase 2 study. Int J Radiat Oncol Biol Phys 85 (1): e55-60, 2013.
- Stupp R, Mason WP, van den Bent MJ, et al.: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352 (10): 987-96, 2005.
- Hegi ME, Diserens AC, Gorlia T, et al.: MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352 (10): 997-1003, 2005.
- Mackay A, Burford A, Molinari V, et al.: Molecular, Pathological, Radiological, and Immune Profiling of Non-brainstem Pediatric High-Grade Glioma from the HERBY Phase II Randomized Trial. Cancer Cell 33 (5): 829-842.e5, 2018.
- Frappaz D, Schell M, Thiesse P, et al.: Preradiation chemotherapy may improve survival in pediatric diffuse intrinsic brainstem gliomas: final results of BSG 98 prospective trial. Neuro Oncol 10 (4): 599-607, 2008.
- Frazier JL, Lee J, Thomale UW, et al.: Treatment of diffuse intrinsic brainstem gliomas: failed approaches and future strategies. J Neurosurg Pediatr 3 (4): 259-69, 2009.
- Hargrave D, Bartels U, Bouffet E: Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7 (3): 241-8, 2006.
- Warren K, Bent R, Wolters PL, et al.: A phase 2 study of pegylated interferon α-2b (PEG-Intron(®)) in children with diffuse intrinsic pontine glioma. Cancer 118 (14): 3607-13, 2012.
- Bouffet E, Raquin M, Doz F, et al.: Radiotherapy followed by high dose busulfan and thiotepa: a prospective assessment of high dose chemotherapy in children with diffuse pontine gliomas. Cancer 88 (3): 685-92, 2000.
- Waters TW, Moore SA, Sato Y, et al.: Refractory infantile high-grade glioma containing TRK-fusion responds to larotrectinib. Pediatr Blood Cancer 68 (5): e28868, 2021.
- Duffner PK, Horowitz ME, Krischer JP, et al.: Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med 328 (24): 1725-31, 1993.
- Nobre L, Zapotocky M, Ramaswamy V, et al.: Outcomes of BRAF V600E Pediatric Gliomas Treated With Targeted BRAF Inhibition. JCO Precis Oncol 4: , 2020.
- Ziegler DS, Wong M, Mayoh C, et al.: Brief Report: Potent clinical and radiological response to larotrectinib in TRK fusion-driven high-grade glioma. Br J Cancer 119 (6): 693-696, 2018.
- Doz F, van Tilburg CM, Geoerger B, et al.: Efficacy and safety of larotrectinib in TRK fusion-positive primary central nervous system tumors. Neuro Oncol 24 (6): 997-1007, 2022.
- Bouffet E, Larouche V, Campbell BB, et al.: Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol 34 (19): 2206-11, 2016.
Treatment of Recurrent Pediatric-Type Diffuse High-Grade Gliomas
To determine and implement optimal management, treatment is best guided by a multidisciplinary team of specialists experienced in treating pediatric patients with brain tumors.
Treatment options for recurrent pediatric-type diffuse high-grade gliomas include the following:
- Second surgery.
- Radiation therapy.
- Targeted therapy.
- Immunotherapy.
Second Surgery
The use of surgical intervention must be individualized on the basis of the following:
- Initial tumor type.
- Length of time between initial treatment and the reappearance of the mass lesion.
- Location of the recurrent tumor.
- Consideration of therapeutics based on the requirement for fresh tumor tissue or to deliver therapy to the operative bed.
- In most cases of diffuse midline gliomas centered in the pons (diffuse intrinsic pontine glioma [DIPG]), biopsy at the time of clinical or radiological progression is neither necessary nor recommended. Biopsy may be considered for confirmation of relapse when treatment-related brain stem damage, which may be clinically indistinguishable from tumor recurrence, is in the differential diagnosis. Other tests, including positron emission tomography, magnetic resonance spectroscopy, and single-photon emission computed tomography, are not reliable in distinguishing necrosis from tumor recurrence in previously irradiated patients with DIPG.
Radiation Therapy
Radiation therapy is appropriate for patients who have not previously been irradiated. Radiation doses and volumes are similar to those used for newly diagnosed patients. Generally, this is limited to young children initially treated with radiation-avoiding strategies.
For previously irradiated patients with non–brain stem pediatric-type high-grade gliomas, reirradiation has been used, although the data demonstrating benefit are sparse. Stereotactic radiosurgery (SRS) or stereotactic radiation therapy (SRT) techniques using either hypofractionated radiation therapy or standard fraction sizes may be considered. For small volume distinct lesions, SRS allows for maximum sparing of normal tissues. For more infiltrative lesions, fractionated radiation therapy may better spare normal tissues.[1]
For patients with DIPG, reirradiation has been shown to prolong survival and can be considered at progression in children who have had an initial response to radiation therapy.[2,3] In a phase I/II study of 12 patients treated at three dose levels (24 Gy/12 fractions, 26.5 Gy/12 fractions, or 30.8 Gy/14 fractions), almost all patients improved. Clinical utility analysis showed that the 24-Gy regimen was preferable.[4] A recent survey confirms the effective use of even lower doses (e.g., 12 Gy fractionated). These doses are beneficial, and they allow for additional radiation therapy courses.[5]
Targeted Therapy
Somatic BRAF V600E variants are present in a small subset of patients. While many of these tumors are responsive to BRAF and/or MEK inhibitors, responses in the recurrent setting are typically not sustained long term. A median progression-free survival of approximately 3 months was reported in one retrospective series.[6] In a multicenter, open-label, single-arm, phase II trial that evaluated dabrafenib plus trametinib, 15 of 45 adult patients with BRAF V600E variants and high-grade gliomas had an objective response. There were three complete responses and 12 partial responses, with a median overall survival of 17.6 months.[7]
The U.S. Food and Drug Administration (FDA) approved the combination of dabrafenib (BRAF inhibitor) plus trametinib (MEK inhibitor) for adult and pediatric patients aged 1 year and older with unresectable or metastatic solid tumors with BRAF V600E variants who have progressed following prior treatment and have no satisfactory alternative treatment options.[8,9] This approval includes pediatric patients aged 1 year and older with BRAF V600E variants and high-grade gliomas. The approval for this patient population was based on the results described below:[8,9,10]
- The dabrafenib-plus-trametinib combination was studied in 41 pediatric patients with relapsed or progressive high-grade gliomas.
- The median age of enrolled patients was 13 years.
- The objective response rate was 56% (95% confidence interval [CI], 39.7%–71.5%).
- For the 23 patients who achieved objective responses, 48% of patients had a duration of response of 12 months and longer and 22% of patients had a duration of response of 24 months or longer.
Activating gene fusions (ALK, NTRK1, NTRK2, NTRK3, ROS1, and MET) are characteristic of infant-type diffuse gliomas.[11,12] Data from case reports and recent prospective clinical trials suggest that these tumors are highly responsive to targeted therapies.[13]
Tumor sample sequencing was done in pediatric (n = 54) and young adult patients (n = 15) with recurrent or refractory high-grade gliomas who were enrolled in the National Cancer Institute (NCI)–Children's Oncology Group (COG) Pediatric MATCH trial. The test revealed genomic alterations that were considered actionable for treatment on MATCH study arms in 36 of 69 tumors (52.2%).[14] Alterations in MAPK pathway genes were detected in 17 of 69 tumors (24.6%), most frequently BRAF V600E variants or fusions (n = 11, 15.9%). FGFR1 variants or fusions were identified in 6 of 69 tumors (8.7%).
Immunotherapy
Numerous studies are investigating a variety of immunotherapy strategies, including checkpoint inhibitors, oncolytic viruses, chimeric antigen receptor (CAR) T cells, and other immune-modulating strategies. The utility of such strategies in the treatment of patients with recurrent pediatric-type diffuse high-grade gliomas is under study, with preliminary evidence of activity in some settings.[15,16]
Treatment Options Under Clinical Evaluation
The role of immune checkpoint inhibition in the treatment of children with recurrent high-grade astrocytoma is currently under study. Children with biallelic mismatch repair deficiency have a very high mutational burden and neoantigen expression and are at risk of developing a variety of cancers, including hematologic malignancies, gastrointestinal cancers, and brain tumors. The high variant and neoantigen load has been correlated with improved response to immune checkpoint inhibition. Early case reports have demonstrated clinical and radiographic responses in children who are treated with an anti–programmed death-1 inhibitor.[17]
Patients for whom initial treatment fails may benefit from additional treatment, including entry into clinical trials of novel therapeutic approaches.[18] Early-phase therapeutic trials may be available for selected patients. These trials may be available via the COG, the Pediatric Brain Tumor Consortium, or other entities. Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified in a patient's tumor (refractory or recurrent). Children and adolescents aged 1 to 21 years are eligible for the trial.
Patients with tumors that have molecular variants addressed by open treatment arms in the trial may be enrolled in treatment on Pediatric MATCH. Additional information can be obtained on the NCI website and ClinicalTrials.gov website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Tsang DS, Oliveira C, Bouffet E, et al.: Repeat irradiation for children with supratentorial high-grade glioma. Pediatr Blood Cancer 66 (9): e27881, 2019.
- Janssens GO, Gandola L, Bolle S, et al.: Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: A matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur J Cancer 73: 38-47, 2017.
- Lassaletta A, Strother D, Laperriere N, et al.: Reirradiation in patients with diffuse intrinsic pontine gliomas: The Canadian experience. Pediatr Blood Cancer 65 (6): e26988, 2018.
- Amsbaugh MJ, Mahajan A, Thall PF, et al.: A Phase 1/2 Trial of Reirradiation for Diffuse Intrinsic Pontine Glioma. Int J Radiat Oncol Biol Phys 104 (1): 144-148, 2019.
- Cacciotti C, Liu KX, Haas-Kogan DA, et al.: Reirradiation practices for children with diffuse intrinsic pontine glioma. Neurooncol Pract 8 (1): 68-74, 2021.
- Nobre L, Zapotocky M, Ramaswamy V, et al.: Outcomes of BRAF V600E Pediatric Gliomas Treated With Targeted BRAF Inhibition. JCO Precis Oncol 4: , 2020.
- Wen PY, Stein A, van den Bent M, et al.: Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol 23 (1): 53-64, 2022.
- Novartis Pharmaceuticals Corporation: TAFINLAR (dabrafenib): Prescribing Information. East Hanover, New Jersey: Novartis Pharmaceuticals Corporation, 2023. Available online. Last accessed February 7, 2024.
- Novartis Pharmaceuticals Corporation: MEKINIST (trametinib): Prescribing Information. East Hanover, New Jersey: Novartis Pharmaceuticals Corporation, 2023. Available online. Last accessed February 7, 2024.
- Hargrave DR, Terashima K, Hara J, et al.: Phase II Trial of Dabrafenib Plus Trametinib in Relapsed/Refractory BRAF V600-Mutant Pediatric High-Grade Glioma. J Clin Oncol 41 (33): 5174-5183, 2023.
- Clarke M, Mackay A, Ismer B, et al.: Infant High-Grade Gliomas Comprise Multiple Subgroups Characterized by Novel Targetable Gene Fusions and Favorable Outcomes. Cancer Discov 10 (7): 942-963, 2020.
- Guerreiro Stucklin AS, Ryall S, Fukuoka K, et al.: Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun 10 (1): 4343, 2019.
- Desai AV, Robinson GW, Gauvain K, et al.: Entrectinib in children and young adults with solid or primary CNS tumors harboring NTRK, ROS1, or ALK aberrations (STARTRK-NG). Neuro Oncol 24 (10): 1776-1789, 2022.
- Parsons DW, Janeway KA, Patton DR, et al.: Actionable Tumor Alterations and Treatment Protocol Enrollment of Pediatric and Young Adult Patients With Refractory Cancers in the National Cancer Institute-Children's Oncology Group Pediatric MATCH Trial. J Clin Oncol 40 (20): 2224-2234, 2022.
- Friedman GK, Johnston JM, Bag AK, et al.: Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N Engl J Med 384 (17): 1613-1622, 2021.
- Majzner RG, Ramakrishna S, Yeom KW, et al.: GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603 (7903): 934-941, 2022.
- Bouffet E, Larouche V, Campbell BB, et al.: Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol 34 (19): 2206-11, 2016.
- Warren KE, Gururangan S, Geyer JR, et al.: A phase II study of O6-benzylguanine and temozolomide in pediatric patients with recurrent or progressive high-grade gliomas and brainstem gliomas: a Pediatric Brain Tumor Consortium study. J Neurooncol 106 (3): 643-9, 2012.
Latest Updates to This Summary (06 / 17 / 2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
General Information About Childhood Astrocytomas, Other Gliomas, and Glioneuronal/Neuronal Tumors
Added Das et al. as reference 21.
Added text to state that ROS1 gene fusions have been reported in gliomas occurring in older children and adults. A retrospective meta-analysis that included 40 children older than 1 year revealed that ROS1 gene fusions occurred in diverse glioma histologies, including diffuse high-grade and low-grade gliomas and glioneuronal tumors. Similar to ROS1-altered cases occurring in infants, tumor variants in other known driver genes were rare. However, tumor copy number alterations were more frequent in older children than infants (cited Meredith et al. as reference 30).
Added text to state that a subset of H3 K27-altered tumors will have a BRAF V600E or FGFR1 co-variant. Added text about the results of a retrospective study that demonstrated a somewhat higher propensity for a thalamic location (cited Auffret et al. as reference 54). Also added text about the results of a separate retrospective study of pediatric and adult patients with H3 K27-altered gliomas that revealed BRAF V600E variants in 5.8% and FGFR1 variants in 10.9% of patients younger than 20 years (cited Williams et al. as reference 55).
Added text to state that the small minority of patients with diffuse midline gliomas lacking histone H3 variants often show EZHIP overexpression. EZHIP inhibits PRC2 activity, leading to the same loss of H3 K27 trimethylation that is induced by H3 K27M variants (cited Jain et al. as reference 56). Overexpression of EZHIP is likewise observed in posterior fossa type A ependymomas, which also shows loss of H3 K27 methylation (cited Hübner et al. as reference 57).
Added text about the prevalence, presentation, and genomics of IDH1- and IDH2-altered tumors that occur in the pediatric population.
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood astrocytomas, other gliomas, and glioneuronal/neuronal tumors. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Astrocytomas, Other Gliomas, and Glioneuronal/Neuronal Tumors Treatment are:
- Kenneth J. Cohen, MD, MBA (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital)
- Louis S. Constine, MD (James P. Wilmot Cancer Center at University of Rochester Medical Center)
- Karen J. Marcus, MD, FACR (Dana-Farber Cancer Institute/Boston Children's Hospital)
- Roger J. Packer, MD (Children's National Hospital)
- D. Williams Parsons, MD, PhD (Texas Children's Hospital)
- Malcolm A. Smith, MD, PhD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Astrocytomas, Other Gliomas, and Glioneuronal/Neuronal Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/brain/hp/child-astrocytoma-glioma-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389382]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us.
Last Revised: 2024-06-17

