Non-Small Cell Lung Cancer Treatment (PDQ®): Treatment - Health Professional Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
General Information About Non-Small Cell Lung Cancer (NSCLC)
NSCLC is any type of epithelial lung cancer other than small cell lung cancer (SCLC). The most common types of NSCLC are squamous cell carcinoma, large cell carcinoma, and adenocarcinoma, but there are several other types that occur less frequently, and all types can occur in unusual histological variants. Although NSCLCs are associated with cigarette smoke, adenocarcinomas may be found in patients who never smoked.
As a class, NSCLC is usually less sensitive to chemotherapy and radiation therapy than SCLC. Patients with resectable disease may be cured by surgery or surgery followed by chemotherapy. Local control can be achieved with radiation therapy in many patients with unresectable disease, but cure is seen in relatively few patients. Patients with locally advanced unresectable disease may achieve long-term survival with radiation therapy combined with chemotherapy. Patients with advanced metastatic disease may achieve improved survival and palliation of symptoms with chemotherapy, targeted agents, and other supportive measures.
Incidence and Mortality
Estimated new cases and deaths from lung cancer (NSCLC and SCLC combined) in the United States in 2024:[1]
- New cases: 234,580.
- Deaths: 125,070.
Lung cancer is the leading cause of cancer-related mortality in the United States. The 5-year relative survival rate from 2013 to 2019 for patients with lung cancer was 25%. The 5-year relative survival rate varies markedly for patients diagnosed at local stage (63%), regional stage (35%), or distant stage (8%).[1]
Anatomy
NSCLC arises from the epithelial cells of the lung of the central bronchi to terminal alveoli. The histological type of NSCLC correlates with site of origin, reflecting the variation in respiratory tract epithelium of the bronchi to alveoli. Squamous cell carcinoma usually starts near a central bronchus. Adenocarcinoma and bronchioloalveolar carcinoma usually originate in peripheral lung tissue.
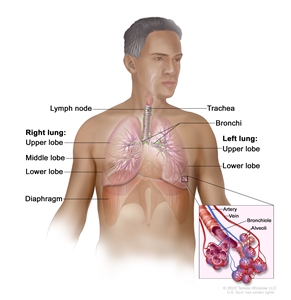
Anatomy of the respiratory system.
Pathogenesis
Smoking-related lung carcinogenesis is a multistep process. Squamous cell carcinoma and adenocarcinoma have defined premalignant precursor lesions. Before becoming invasive, lung epithelium may undergo morphological changes that include:
- Hyperplasia.
- Metaplasia.
- Dysplasia.
- Carcinoma in situ.
Dysplasia and carcinoma in situ are considered the principal premalignant lesions because they are more likely to progress to invasive cancer and less likely to spontaneously regress.
Risk Factors
Increasing age is the most important risk factor for most cancers. Other risk factors for lung cancer include the following:
- History of or current tobacco use: cigarettes, pipes, and cigars.[2]
- Exposure to cancer-causing substances in secondhand smoke.[3,4]
- Occupational exposure to asbestos, arsenic, chromium, beryllium, nickel, and other agents.[5]
- Radiation exposure from any of the following:
- Living in an area with air pollution.[10,11,12]
- Family history of lung cancer.[13]
- Human immunodeficiency virus infection.[14]
- Beta carotene supplements in heavy smokers.[15,16]
The single most important risk factor for the development of lung cancer is smoking. For a smoker, the risk of lung cancer is, on average, tenfold higher than in a lifetime nonsmoker (defined as a person who has smoked <100 cigarettes in his or her lifetime). The risk increases with the quantity of cigarettes, duration of smoking, and starting age.
Smoking cessation results in a decrease in precancerous lesions and a reduction in lung cancer risk. Former smokers continue to have an elevated risk of lung cancer for years after quitting. Asbestos exposure may exert a synergistic effect of cigarette smoking on lung cancer risk.[17]
In addition, after resection of a lung cancer, there is a 1% to 2% risk per patient per year that a second lung cancer will occur.[18]
A significant number of patients cured of their smoking-related lung cancer may develop a second malignancy. In the Lung Cancer Study Group trial of 907 patients with stage T1, N0 resected tumors, the rate was 1.8% per year for nonpulmonary second cancers and 1.6% per year for new lung cancers.[19] Other studies have reported even higher risks of second tumors in long-term survivors, including rates of 10% for second lung cancers and 20% for all second cancers.[20]
Because of the persistent risk of developing second lung cancers in former smokers, various chemoprevention strategies have been evaluated in randomized control trials. None of the phase III trials using the agents beta carotene, retinol, 13-cis -retinoic acid, [alpha]-tocopherol, N-acetylcysteine, or acetylsalicylic acid has demonstrated beneficial, reproducible results.[16,21,22,23,24][Level of evidence A1] Chemoprevention of second primary cancers of the upper aerodigestive tract is undergoing clinical evaluation in patients with early-stage lung cancer.
For more information, see Lung Cancer Prevention.
Screening
In patients considered at high risk of developing lung cancer, the only screening modality for early detection that has been shown to alter mortality is low-dose helical CT scanning.[25] Studies have failed to demonstrate that screening with chest radiography and sputum cytology lowers lung cancer mortality rates.
For more information, see the Screening by low-dose computed tomography: benefit section in Lung Cancer Screening.
Clinical Presentation
Lung cancer may present with symptoms or be found incidentally on chest imaging. The most common symptoms at presentation include:
- Worsening cough.
- Chest pain.
- Hemoptysis.
- Malaise.
- Weight loss.
- Dyspnea.
- Hoarseness.
Symptoms may result from local invasion or compression of adjacent thoracic structures, such as compression involving the esophagus causing dysphagia, compression involving the laryngeal nerves causing hoarseness, or compression involving the superior vena cava causing facial edema and distension of the superficial veins of the head and neck.
Symptoms from distant metastases may also be present and include neurological defect or personality change from brain metastases or pain from bone metastases. Infrequently, patients may present with symptoms and signs of paraneoplastic diseases such as hypertrophic osteoarthropathy with digital clubbing or hypercalcemia from parathyroid hormone-related protein.
Physical examination may identify enlarged supraclavicular lymphadenopathy, pleural effusion or lobar collapse, unresolved pneumonia, or signs of associated disease such as chronic obstructive pulmonary disease or pulmonary fibrosis.
Diagnosis
Investigations of patients with suspected NSCLC focus on confirming the diagnosis and determining the extent of the disease. Treatment options are determined by histology, stage, and general health and comorbidities of the patient.
The procedures used to determine the presence of cancer include:
- History.
- Physical examination.
- Routine laboratory evaluations.
- Chest x-ray.
- Chest CT scan with infusion of contrast material.
- Biopsy.
Before a patient begins lung cancer treatment, an experienced lung cancer pathologist must review the pathological material. This is critical because SCLC, which responds well to chemotherapy and is generally not treated surgically, can be confused on microscopic examination with NSCLC.[26] Immunohistochemistry and electron microscopy are invaluable techniques for diagnosis and subclassification, but most lung tumors can be classified by light microscopic criteria.
For more information on tests and procedures used for staging, see the General Staging Evaluation section.
Prognostic Factors
Multiple studies have attempted to identify the prognostic importance of a variety of clinicopathological factors.[20,27,28,29,30] Factors that have correlated with adverse prognosis include:
- Increasing stage.
- Presence of pulmonary or constitutional symptoms.
- Large tumor size (>3 cm).
- Metastases to multiple lymph nodes within a TNM-defined nodal station.[31,32,33,34,35,36,37,38,39,40,41] For more information, see the Evaluation of mediastinal lymph node metastasis section.
- Vascular invasion.[28,42,43,44]
For patients with inoperable disease, prognosis is adversely affected by poor performance status and weight loss of more than 10%. These patients have been excluded from clinical trials evaluating aggressive multimodality interventions.
In multiple retrospective analyses of clinical trial data, advanced age alone has not been shown to influence response or survival with therapy.[45]
Because treatment is not satisfactory for almost all patients with NSCLC, eligible patients should consider clinical trials. Information about ongoing clinical trials is available from the NCI website.
References:
- American Cancer Society: Cancer Facts and Figures 2024. American Cancer Society, 2024. Available online. Last accessed June 21, 2024.
- Alberg AJ, Ford JG, Samet JM, et al.: Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132 (3 Suppl): 29S-55S, 2007.
- Tulunay OE, Hecht SS, Carmella SG, et al.: Urinary metabolites of a tobacco-specific lung carcinogen in nonsmoking hospitality workers. Cancer Epidemiol Biomarkers Prev 14 (5): 1283-6, 2005.
- Anderson KE, Kliris J, Murphy L, et al.: Metabolites of a tobacco-specific lung carcinogen in nonsmoking casino patrons. Cancer Epidemiol Biomarkers Prev 12 (12): 1544-6, 2003.
- Straif K, Benbrahim-Tallaa L, Baan R, et al.: A review of human carcinogens--part C: metals, arsenic, dusts, and fibres. Lancet Oncol 10 (5): 453-4, 2009.
- Friedman DL, Whitton J, Leisenring W, et al.: Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 102 (14): 1083-95, 2010.
- Gray A, Read S, McGale P, et al.: Lung cancer deaths from indoor radon and the cost effectiveness and potential of policies to reduce them. BMJ 338: a3110, 2009.
- Berrington de González A, Kim KP, Berg CD: Low-dose lung computed tomography screening before age 55: estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J Med Screen 15 (3): 153-8, 2008.
- Shimizu Y, Kato H, Schull WJ: Studies of the mortality of A-bomb survivors. 9. Mortality, 1950-1985: Part 2. Cancer mortality based on the recently revised doses (DS86). Radiat Res 121 (2): 120-41, 1990.
- Katanoda K, Sobue T, Satoh H, et al.: An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J Epidemiol 21 (2): 132-43, 2011.
- Cao J, Yang C, Li J, et al.: Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study. J Hazard Mater 186 (2-3): 1594-600, 2011.
- Hales S, Blakely T, Woodward A: Air pollution and mortality in New Zealand: cohort study. J Epidemiol Community Health 66 (5): 468-73, 2012.
- Lissowska J, Foretova L, Dabek J, et al.: Family history and lung cancer risk: international multicentre case-control study in Eastern and Central Europe and meta-analyses. Cancer Causes Control 21 (7): 1091-104, 2010.
- Shiels MS, Cole SR, Kirk GD, et al.: A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr 52 (5): 611-22, 2009.
- The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 330 (15): 1029-35, 1994.
- Omenn GS, Goodman GE, Thornquist MD, et al.: Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 334 (18): 1150-5, 1996.
- Wingo PA, Ries LA, Giovino GA, et al.: Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst 91 (8): 675-90, 1999.
- Johnson BE: Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 90 (18): 1335-45, 1998.
- Thomas P, Rubinstein L: Cancer recurrence after resection: T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 49 (2): 242-6; discussion 246-7, 1990.
- Martini N, Bains MS, Burt ME, et al.: Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 109 (1): 120-9, 1995.
- van Boxem AJ, Westerga J, Venmans BJ, et al.: Photodynamic therapy, Nd-YAG laser and electrocautery for treating early-stage intraluminal cancer: which to choose? Lung Cancer 31 (1): 31-6, 2001.
- Blumberg J, Block G: The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finland. Nutr Rev 52 (7): 242-5, 1994.
- Lippman SM, Lee JJ, Karp DD, et al.: Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst 93 (8): 605-18, 2001.
- van Zandwijk N, Dalesio O, Pastorino U, et al.: EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the EUropean Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst 92 (12): 977-86, 2000.
- Aberle DR, Adams AM, Berg CD, et al.: Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365 (5): 395-409, 2011.
- Travis WD, Colby TV, Corrin B, et al.: Histological typing of lung and pleural tumours. 3rd ed. Springer-Verlag, 1999.
- Albain KS, Crowley JJ, LeBlanc M, et al.: Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 9 (9): 1618-26, 1991.
- Macchiarini P, Fontanini G, Hardin MJ, et al.: Blood vessel invasion by tumor cells predicts recurrence in completely resected T1 N0 M0 non-small-cell lung cancer. J Thorac Cardiovasc Surg 106 (1): 80-9, 1993.
- Ichinose Y, Yano T, Asoh H, et al.: Prognostic factors obtained by a pathologic examination in completely resected non-small-cell lung cancer. An analysis in each pathologic stage. J Thorac Cardiovasc Surg 110 (3): 601-5, 1995.
- Fontanini G, Bigini D, Vignati S, et al.: Microvessel count predicts metastatic disease and survival in non-small cell lung cancer. J Pathol 177 (1): 57-63, 1995.
- Sayar A, Turna A, Kiliçgün A, et al.: Prognostic significance of surgical-pathologic multiple-station N1 disease in non-small cell carcinoma of the lung. Eur J Cardiothorac Surg 25 (3): 434-8, 2004.
- Osaki T, Nagashima A, Yoshimatsu T, et al.: Survival and characteristics of lymph node involvement in patients with N1 non-small cell lung cancer. Lung Cancer 43 (2): 151-7, 2004.
- Ichinose Y, Kato H, Koike T, et al.: Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: questionnaire survey of the Japan Clinical Oncology Group to plan for clinical trials. Lung Cancer 34 (1): 29-36, 2001.
- Tanaka F, Yanagihara K, Otake Y, et al.: Prognostic factors in patients with resected pathologic (p-) T1-2N1M0 non-small cell lung cancer (NSCLC). Eur J Cardiothorac Surg 19 (5): 555-61, 2001.
- Asamura H, Suzuki K, Kondo H, et al.: Where is the boundary between N1 and N2 stations in lung cancer? Ann Thorac Surg 70 (6): 1839-45; discussion 1845-6, 2000.
- Riquet M, Manac'h D, Le Pimpec-Barthes F, et al.: Prognostic significance of surgical-pathologic N1 disease in non-small cell carcinoma of the lung. Ann Thorac Surg 67 (6): 1572-6, 1999.
- van Velzen E, Snijder RJ, Brutel de la Rivière A, et al.: Lymph node type as a prognostic factor for survival in T2 N1 M0 non-small cell lung carcinoma. Ann Thorac Surg 63 (5): 1436-40, 1997.
- Vansteenkiste JF, De Leyn PR, Deneffe GJ, et al.: Survival and prognostic factors in resected N2 non-small cell lung cancer: a study of 140 cases. Leuven Lung Cancer Group. Ann Thorac Surg 63 (5): 1441-50, 1997.
- Izbicki JR, Passlick B, Karg O, et al.: Impact of radical systematic mediastinal lymphadenectomy on tumor staging in lung cancer. Ann Thorac Surg 59 (1): 209-14, 1995.
- Martini N, Burt ME, Bains MS, et al.: Survival after resection of stage II non-small cell lung cancer. Ann Thorac Surg 54 (3): 460-5; discussion 466, 1992.
- Naruke T, Goya T, Tsuchiya R, et al.: Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg 96 (3): 440-7, 1988.
- Thomas P, Doddoli C, Thirion X, et al.: Stage I non-small cell lung cancer: a pragmatic approach to prognosis after complete resection. Ann Thorac Surg 73 (4): 1065-70, 2002.
- Macchiarini P, Fontanini G, Hardin MJ, et al.: Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet 340 (8812): 145-6, 1992.
- Khan OA, Fitzgerald JJ, Field ML, et al.: Histological determinants of survival in completely resected T1-2N1M0 nonsmall cell cancer of the lung. Ann Thorac Surg 77 (4): 1173-8, 2004.
- Earle CC, Tsai JS, Gelber RD, et al.: Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. J Clin Oncol 19 (4): 1064-70, 2001.
Cellular and Molecular Classification of NSCLC
Malignant non-small cell epithelial tumors of the lung are classified by the World Health Organization (WHO)/International Association for the Study of Lung Cancer (IASLC). The three main subtypes of non-small cell lung cancer (NSCLC) include:
- Squamous cell carcinoma (25% of lung cancers).
- Adenocarcinoma (40% of lung cancers).
- Large cell carcinoma (10% of lung cancers).
Additional types include adenosquamous carcinoma, sarcomatoid carcinomas, salivary gland type tumors, carcinoid tumors, and other unclassified carcinomas. There are many subtypes in these categories.[1]
Tumor Types
Squamous cell carcinoma
Most squamous cell carcinomas of the lung are located centrally, in the larger bronchi of the lung. Squamous cell carcinomas are linked more strongly with smoking than other forms of NSCLC. The incidence of squamous cell carcinoma of the lung has been decreasing in recent years.
Adenocarcinoma
Adenocarcinoma is now the most common histological subtype in many countries, and subclassification of adenocarcinoma is important. One of the biggest problems with lung adenocarcinomas is the frequent histological heterogeneity. Mixtures of adenocarcinoma histological subtypes are more common than tumors consisting purely of a single pattern of acinar, papillary, bronchioloalveolar, and solid adenocarcinoma with mucin formation.
Criteria for the diagnosis of bronchioloalveolar carcinoma have varied widely in the past. The current WHO/IASLC definition is much more restrictive than that previously used by many pathologists because it is limited to only noninvasive tumors.
If stromal, vascular, or pleural invasion are identified in an adenocarcinoma that has an extensive bronchioloalveolar carcinoma component, the classification would be an adenocarcinoma of mixed subtype with predominant bronchioloalveolar pattern and a focal acinar, solid, or papillary pattern, depending on which pattern is seen in the invasive component. However, the future of bronchioloalveolar carcinoma as a distinct clinical entity is unclear; a multidisciplinary expert panel representing the IASLC, the American Thoracic Society, and the European Respiratory Society proposed a major revision of the classification of adenocarcinomas in 2011 that entails a reclassification of what was called bronchioloalveolar carcinoma into newly defined histological subgroups.
The following variants of adenocarcinoma are recognized in the WHO/IASLC classification:
- Well-differentiated fetal adenocarcinoma.
- Mucinous (colloid) adenocarcinoma.
- Mucinous cystadenocarcinoma.
- Signet ring adenocarcinoma.
- Clear cell adenocarcinoma.
Large cell carcinoma
In addition to the general category of large cell carcinoma, several uncommon variants are recognized in the WHO/IASLC classification, including:
- Large cell neuroendocrine carcinoma (LCNEC).
- Basaloid carcinoma.
- Lymphoepithelioma-like carcinoma.
- Clear cell carcinoma.
- Large cell carcinoma with rhabdoid phenotype.
Basaloid carcinoma is also recognized as a variant of squamous cell carcinoma, and rarely, adenocarcinomas may have a basaloid pattern; however, in tumors without either of these features, they are regarded as a variant of large cell carcinoma.
Neuroendocrine tumors
LCNEC is recognized as a histologically high-grade non-small cell carcinoma. It has a very poor prognosis similar to that of small cell lung cancer (SCLC). Atypical carcinoid is recognized as an intermediate-grade neuroendocrine tumor with a prognosis that falls between typical carcinoid and high-grade SCLC and LCNEC.
Neuroendocrine differentiation can be demonstrated by immunohistochemistry or electron microscopy in 10% to 20% of common NSCLCs that do not have any neuroendocrine morphology. These tumors are not formally recognized within the WHO/IASLC classification scheme because the clinical and therapeutic significance of neuroendocrine differentiation in NSCLC is not firmly established. These tumors are referred to collectively as NSCLC with neuroendocrine differentiation.
Carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements
This is a group of rare tumors. Spindle cell carcinomas and giant cell carcinomas comprise only 0.4% of all lung malignancies, and carcinosarcomas comprise only 0.1% of all lung malignancies. In addition, this group of tumors reflects a continuum in histological heterogeneity, as well as epithelial and mesenchymal differentiation. On the basis of clinical and molecular data, biphasic pulmonary blastoma is regarded as part of the spectrum of carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements.
Molecular Features
The identification of mutations in lung cancer has led to the development of molecularly targeted therapy to improve the survival of subsets of patients with metastatic disease.[2] In particular, subsets of adenocarcinoma now can be defined by specific mutations in genes encoding components of the epidermal growth factor receptor (EGFR) and downstream mitogen-activated protein kinases (MAPK) and phosphatidylinositol 3-kinases (PI3K) signaling pathways. These mutations may define mechanisms of drug sensitivity and primary or acquired resistance to kinase inhibitors. Genomic alterations that can be targeted with approved therapies or for which treatments are under development include:
- EGFR.
- ALK.
- BRAF.
- ROS1.
- RET.
- NTRK1, NTRK2, and NTRK3.
- MET.
- KRAS.
- HER2.
EGFR and ALK mutations predominate in adenocarcinomas that develop in nonsmokers, and KRAS and BRAF mutations are more common in smokers or former smokers. EGFR mutations strongly predict the improved response rate and progression-free survival of patients who take EGFR inhibitors. In a set of 2,142 lung adenocarcinoma specimens from patients treated at Memorial Sloan Kettering Cancer Center, EGFR exon 19 deletions and L858R were found in 15% of tumors from former smokers (181 of 1,218; 95% confidence interval [CI], 13%–17%), 6% from current smokers (20 of 344; 95% CI, 4%–9%), and 52% from never-smokers (302 of 580; 95% CI, 48%–56%; P < .001 for ever- vs. never-smokers).[3]
Fusions of ALK with EML4 genes form translocation products that occur in ranges from 3% to 7% in unselected NSCLC and are responsive to pharmacological inhibition of ALK by agents such as crizotinib. Sensitizing fusions of ALK with other genes have also been reported.
References:
- Travis WD, Brambilla E, Nicholson AG, et al.: The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 10 (9): 1243-1260, 2015.
- Pao W, Girard N: New driver mutations in non-small-cell lung cancer. Lancet Oncol 12 (2): 175-80, 2011.
- D'Angelo SP, Pietanza MC, Johnson ML, et al.: Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol 29 (15): 2066-70, 2011.
Stage Information for NSCLC
General Staging Evaluation
In non-small cell lung cancer (NSCLC), the determination of stage has important therapeutic and prognostic implications. Careful initial diagnostic evaluation to define the location and to determine the extent of primary and metastatic tumor involvement is critical for the appropriate care of patients.
In general, symptoms, physical signs, laboratory findings, and perceived risk of distant metastasis lead to an evaluation for distant metastatic disease. Additional tests such as bone scans and computed tomography (CT)/magnetic resonance imaging (MRI) of the brain may be performed if initial assessments suggest metastases or if patients with stage III disease are being evaluated for aggressive local and combined modality treatments.
Stage has a critical role in the selection of therapy. The stage of disease is based on a combination of clinical factors and pathological factors.[1] The distinction between clinical stage and pathological stage should be considered when evaluating reports of survival outcome.
Procedures used to determine stage include:
- History.
- Physical examination.
- Routine laboratory evaluations.
- Chest x-ray.
- Chest CT scan with infusion of contrast material.
- Fluorine F 18-fludeoxyglucose positron emission tomography (18F-FDG PET) scanning.
Procedures used to obtain tissue samples include bronchoscopy, mediastinoscopy, or anterior mediastinotomy.
Pathological staging of NSCLC requires examination of the tumor, knowledge of resection margins, and determination of lymph node status.
At diagnosis, patients with NSCLC can be divided into the following three groups that reflect both the extent of the disease and the treatment approach:
- Surgically resectable disease (generally stage I, stage II, and selected stage III tumors).
- Has the best prognosis, which depends on a variety of tumor and host factors.
- Patients with resectable disease who have medical contraindications to surgery are candidates for curative radiation therapy.
- Postoperative cisplatin-based combination chemotherapy may provide a survival advantage for patients with resected stage II or stage IIIA NSCLC.
- Locally (T3–T4) and/or regionally (N2–N3) advanced disease.
- Has a diverse natural history.
- Selected patients with locally advanced tumors may benefit from combined modality treatments.
- Patients with unresectable or N2–N3 disease are treated with radiation therapy in combination with chemotherapy.
- Selected patients with T3 or N2 disease can be treated effectively with surgical resection and either preoperative or postoperative chemotherapy or chemoradiation therapy.
- Distant metastatic disease (includes distant metastases [M1] that were found at the time of diagnosis).
- May be treated with systemic therapy (chemotherapy and/or immunotherapy or targeted therapy). Radiation therapy can be used for palliation.
Evaluation of mediastinal lymph node metastasis
Surgical evaluation
Surgical staging of the mediastinum is considered standard if accurate evaluation of the nodal status is needed to determine therapy.
Accurate staging of the mediastinal lymph nodes provides important prognostic information.
Evidence (nodal status):
- The association between survival and the number of examined lymph nodes during surgery for patients with stage I NSCLC treated with definitive surgical resection was assessed from the population-based Surveillance, Epidemiology, and End Results (SEER) Program database for the period from 1990 to 2000.[2] A total of 16,800 patients were included in the study.
- The overall survival analysis for patients without radiation therapy was done by comparing the reference group (one to four lymph nodes) with the following groups:
- Patients with five to eight lymph nodes examined during surgery had a modest but statistically significant increase in survival, with a proportionate hazard ratio (HR) of 0.90 (95% confidence interval [CI], 0.84–0.97).
- For patients with 9 to 12 examined lymph nodes, the HR was 0.86 (95% CI, 0.79–0.95).
- For patients with 13 to 16 examined lymph nodes, the HR was 0.78 (95% CI, 0.68–0.90).
- There appeared to be no incremental improvement after evaluating more than 16 lymph nodes.
The corresponding results for lung cancer–specific mortality and for patients who received radiation therapy were not substantially different.
- These results indicate that patient survival following resection for NSCLC is associated with the number of lymph nodes evaluated during surgery. Because this is most likely the result of a reduction-of-staging error, namely, a decreased likelihood of missing positive lymph nodes with an increasing number of lymph nodes sampled, it suggests that an evaluation of nodal status should include 11 to 16 lymph nodes.
- The overall survival analysis for patients without radiation therapy was done by comparing the reference group (one to four lymph nodes) with the following groups:
CT imaging
CT scanning is primarily used for determining the size of the tumor. The CT scan should extend inferiorly to include the liver and adrenal glands. MRI scans of the thorax and upper abdomen do not appear to yield advantages over CT scans.[3]
Evidence (CT scan):
- A systematic review of the medical literature relating to the accuracy of CT scanning for noninvasive staging of the mediastinum in patients with lung cancer was conducted. In the 35 studies published between 1991 and June 2006, 5,111 evaluable patients were identified. Almost all studies specified that CT scanning was performed following the administration of intravenous contrast material and that a positive test result was defined as the presence of one or more lymph nodes that measured larger than 1 cm on the short-axis diameter.[4]
- The median prevalence of mediastinal metastasis was 28% (range, 18%–56%).
- The pooled estimates of sensitivity and specificity of CT scanning for identifying mediastinal lymph node metastasis were 51% (95% CI, 47%–54%) for sensitivity and 86% (95% CI, 84%–88%) for specificity. Corresponding positive (3.4%) and negative (0.6%) likelihood ratios were provided.
- The results from the systematic review are similar to those of a large meta-analysis that reported the median sensitivity and specificity of CT scanning for identifying malignant mediastinal nodes as 61% for sensitivity and 79% for specificity.[5]
- An earlier meta-analysis reported an average sensitivity rate of 64% and specificity rate of 74%.[6]
18F-FDG PET scanning
The wider availability and use of 18F-FDG PET scanning for staging has modified the approach to staging mediastinal lymph nodes and distant metastases.
Randomized trials evaluating the utility of 18F-FDG PET scanning in potentially resectable NSCLC patients reported conflicting results in terms of the relative reduction in the number of noncurative thoracotomies.
Although the current evidence is conflicting, 18F-FDG PET scanning may improve results of early-stage lung cancer by identifying patients who have evidence of metastatic disease that is beyond the scope of surgical resection and that is not evident by standard preoperative staging procedures.
Evidence (18F-FDG PET scan):
- A systematic review, an expansion of a health technology assessment conducted in 2001 by the Institute for Clinical and Evaluative Sciences, evaluated the accuracy and utility of 18F-FDG PET scanning in the diagnosis and staging of lung cancer.[7] Through a systematic search of the literature, 12 evidence summary reports and 15 prospective studies of the diagnostic accuracy of 18F-FDG PET scanning were identified.
- 18F-FDG PET scanning appears to be superior to CT imaging for mediastinal staging in NSCLC.
- 18F-FDG PET scanning also appears to have high sensitivity and reasonable specificity for differentiating benign from malignant lesions as small as 1 cm.
- A systematic review of the medical literature relating to the accuracy of 18F-FDG PET scanning for noninvasive staging of the mediastinum in patients with lung cancer identified 44 studies published between 1994 and 2006 with 2,865 evaluable patients.[4]
- The median prevalence of mediastinal metastases was 29% (range, 5%–64%).
- Pooled estimates of sensitivity and specificity for identifying mediastinal metastasis were 74% (95% CI, 69%–79%) for sensitivity and 85% (95% CI, 82%–88%) for specificity.
- Corresponding positive (4.9%) and negative (0.3%) likelihood ratios were provided for mediastinal staging with 18F-FDG PET scanning.
- These findings demonstrated that 18F-FDG PET scanning is more accurate than CT scanning for staging of the mediastinum in patients with lung cancer.
Decision analyses demonstrate that 18F-FDG PET scanning may reduce the overall costs of medical care by identifying patients with falsely negative CT scans in the mediastinum or otherwise undetected sites of metastases.[8,9,10] Studies concluded that the money saved by forgoing mediastinoscopy in 18F-FDG PET-positive mediastinal lesions was not justified because of the unacceptably high number of false-positive results.[8,9,10] A randomized study found that the addition of 18F-FDG PET scanning to conventional staging was associated with significantly fewer thoracotomies.[11] A second randomized trial evaluating the impact of 18F-FDG PET scanning on clinical management found that 18F-FDG PET scanning provided additional information regarding appropriate stage but did not lead to significantly fewer thoracotomies.[12]
Combination of CT imaging and 18F-FDG PET scanning
The combination of CT imaging and 18F-FDG PET scanning has greater sensitivity and specificity than CT imaging alone.[13]
Evidence (CT/18F-FDG PET scan):
- If there is no evidence of distant metastatic disease on CT scan, 18F-FDG PET scanning complements CT scan staging of the mediastinum. Numerous nonrandomized studies of 18F-FDG PET scanning have evaluated mediastinal lymph nodes using surgery (i.e., mediastinoscopy and/or thoracotomy with mediastinal lymph node dissection) as the gold standard of comparison.
- A meta-analysis evaluated the conditional test performance of 18F-FDG PET scanning and CT scanning.
- The median sensitivity and specificity of 18F-FDG PET scans were reported as 100% for sensitivity and 78% for specificity in patients with enlarged lymph nodes.[5]
- 18F-FDG PET scanning is considered very accurate in identifying malignant nodal involvement when lymph nodes are enlarged. However, 18F-FDG PET scanning will falsely identify a malignancy in approximately one-fourth of patients with lymph nodes that are enlarged for other reasons, usually as a result of inflammation or infection.[14,15]
- The median sensitivity and specificity of 18F-FDG PET scanning in patients with normal-sized mediastinal lymph nodes were 82% for sensitivity and 93% for specificity.[5] These data indicate that nearly 20% of patients with normal-sized lymph nodes but with malignant involvement had falsely negative 18F-FDG PET scan findings.
For patients with clinically operable NSCLC, the evidence supports performing a biopsy of mediastinal lymph nodes that are found to be larger than 1 cm in shortest transverse axis on chest CT scan or are found to be positive on 18F-FDG PET scan. Negative 18F-FDG PET scanning does not preclude biopsy of radiographically enlarged mediastinal lymph nodes. Mediastinoscopy is necessary for the detection of cancer in mediastinal lymph nodes when the results of the CT scan and 18F-FDG PET scan do not corroborate each other.
Evaluation of brain metastasis
Patients at risk of brain metastases may be staged with CT or MRI scans.
Evidence (staging with CT or MRI):
- One study randomly assigned 332 patients with potentially operable NSCLC and no neurological symptoms to brain CT or MRI imaging to detect occult brain metastasis before lung surgery.[16]
- MRI showed a trend towards a higher preoperative detection rate than CT scan (P = .069), with an overall detection rate of approximately 7% from pretreatment to 12 months after surgery.
- Patients with stage I or stage II disease had a detection rate of 4% (i.e., 8 detections out of 200 patients); however, individuals with stage III disease had a detection rate of 11.4% (i.e., 15 detections out of 132 patients).
- The mean maximal diameter of the brain metastases was significantly smaller in the MRI group.
Whether the improved detection rate of MRI translates into improved outcome remains unknown. Not all patients are able to tolerate MRI, and for these patients contrast-enhanced CT scan is a reasonable substitute.
Evaluation of distant metastasis to sites other than the brain
Numerous nonrandomized, prospective, and retrospective studies have demonstrated that 18F-FDG PET scanning offers diagnostic advantages over conventional imaging in staging distant metastatic disease; however, standard 18F-FDG PET scans have limitations. 18F-FDG PET scans may not extend below the pelvis and may not detect bone metastases in the long bones of the lower extremities. Because the metabolic tracer used in 18F-FDG PET scanning accumulates in the brain and urinary tract, 18F-FDG PET scanning is not reliable for detection of metastases in these sites.[16]
The Revised International System for Staging Lung Cancer
The Revised International System for Staging Lung Cancer, based on information from a clinical database of more than 5,000 patients, was adopted in 2010 by the American Joint Committee on Cancer (AJCC) and the Union Internationale Contre le Cancer.[17,18] These revisions provide greater prognostic specificity for patient groups; however, the correlation between stage and prognosis predates the widespread availability of PET imaging.
AJCC Stage Groupings and TNM Definitions
The AJCC has designated staging by TNM (tumor, node, metastasis) classification to define NSCLC.[18]
| T Category | T Criteria | |
|---|---|---|
| a Reprinted with permission from AJCC: Lung. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 431–56. | ||
| TX | Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy. | |
| T0 | No evidence of primary tumor. | |
| Tis | Carcinomain situ; SCIS =Squamous cell carcinomain situ; AIS: Adenocarcinoma in situ; Adenocarcinoma with pure lepidic pattern, ≤3 cm in greatest dimension. | |
| T1 | Tumor ≤3 cm in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus). | |
| T1mi | Minimally invasive adenocarcinoma: adenocarcinoma (≤3 cm in greatest dimension) with a predominantly lepidic pattern and ≤5 mm invasion in greatest dimension. | |
| T1a | Tumor ≤1 cm in greatest dimension. A superficial, spreading tumor of any size whose invasive component is limited to the bronchial wall and may extend proximal to the main bronchus also is classified as T1a, but these tumors are uncommon. | |
| T1b | Tumor >1 cm but ≤2 cm in greatest dimension. | |
| T1c | Tumor >2 cm but ≤3 cm in greatest dimension. | |
| T2 | Tumor >3 cm but ≤5 cm or having any of the following features: involves the main bronchus regardless of distance to the carina, but without involvement of the carina; invades visceral pleura (PL1 or PL2); associated with atelectasis or obstructive pneumonitis that extends to the hilar region, involving part or all of the lung. T2 tumors with these features are classified as T2a if ≤4 cm or if the size cannot be determined and T2b if >4 cm but ≤5 cm. | |
| T2a | Tumor >3 cm but ≤4 cm in greatest dimension. | |
| T2b | Tumor >4 cm but ≤5 cm in greatest dimension. | |
| T3 | Tumor >5 cm but ≤7 cm in greatest dimension or directly invading any of the following: parietal pleura (PL3), chest wall (including superior sulcus tumors), phrenic nerve, parietal pericardium; or separate tumor nodule(s) in the same lobe as the primary. | |
| T4 | Tumor >7 cm or tumor of any size invading one or more of the following: diaphragm, mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, or carina; separate tumor nodule(s) in an ipsilateral lobe different from that of the primary. | |
| N Category | N Criteria |
|---|---|
| a Reprinted with permission from AJCC: Lung. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 431–56. | |
| NX | Regional lymph nodes cannot be assessed. |
| N0 | No regional lymph node metastasis. |
| N1 | Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension. |
| N2 | Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s). |
| N3 | Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s). |
| M Category | M Criteria | |
|---|---|---|
| a Reprinted with permission from AJCC: Lung. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 431–56. | ||
| M0 | No distant metastasis. | |
| M1 | Distant metastasis. | |
| M1a | Separate tumor nodule(s) in a contralateral lobe; tumor with pleural or pericardial nodules or malignant pleural or pericardial effusion. Most pleural (pericardial) effusions with lung cancer are a result of the tumor. In a few patients, however, multiple microscopic examinations of pleural (pericardial) fluid are negative for tumor, and the fluid is nonbloody and not an exudate. If these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging descriptor. | |
| M1b | Single extrathoracic metastases in a single organ (including involvement of a single nonregional node). | |
| M1c | Multiple extrathoracic metastases in a single organ or in multiple organs. | |
| Stage | TNM Classification | Illustration |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| a Reprinted with permission from AJCC: Lung. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 431–56. | ||
| Occult carcinoma | TX, N0, M0 | |
| 0 | Tis, N0, M0 | |
| IA1 | T1mi, N0, M0 | 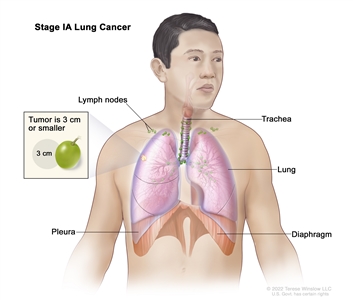 |
| T1a, N0, M0 | ||
| IA2 | T1b, N0, M0 | |
| IA3 | T1c, N0, M0 | |
| IB | T2a, N0, M0 | 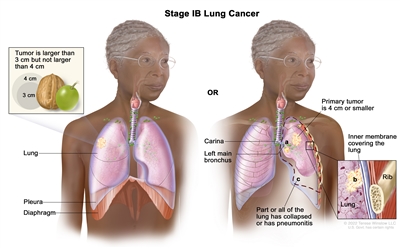 |
| IIA | T2b, N0, M0 | 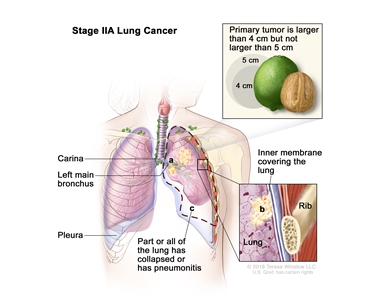 |
| IIB | T1a, N1, M0 | 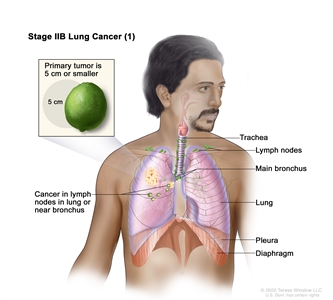 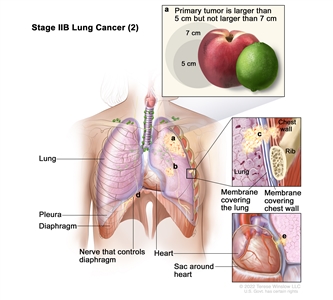 |
| T1b, N1, M0 | ||
| T1c, N1, M0 | ||
| T2a, N1, M0 | ||
| T2b, N1, M0 | ||
| T3, N0, M0 | ||
| IIIA | T1a, N2, M0 | 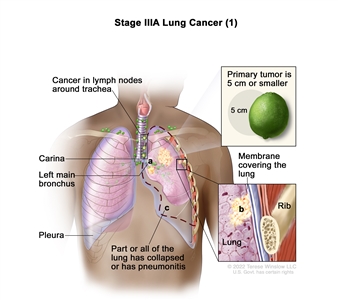 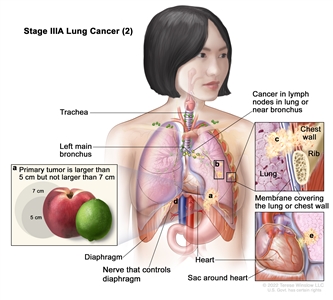 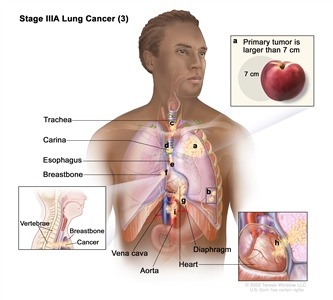 |
| T1b, N2, M0 | ||
| T1c, N2, M0 | ||
| T2a, N2, M0 | ||
| T2b, N2, M0 | ||
| T3, N1, M0 | ||
| T4, N0, M0 | ||
| T4, N1, M0 | ||
| IIIB | T1a, N3, M0 | 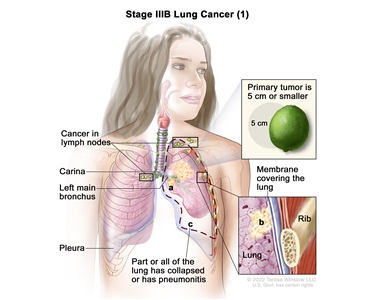 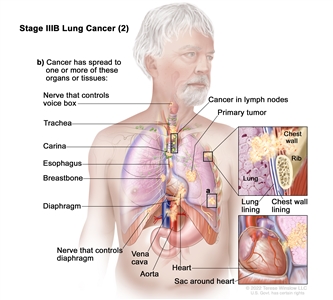 |
| T1b, N3, M0 | ||
| T1c, N3, M0 | ||
| T2a, N3, M0 | ||
| T2b, N3, M0 | ||
| T3, N2, M0 | ||
| T4, N2, M0 | ||
| IIIC | T3, N3, M0 | 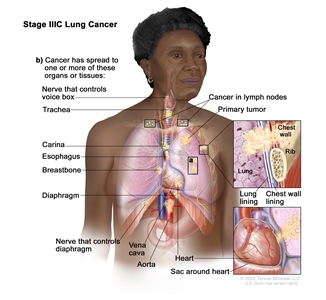 |
| T4, N3, M0 | ||
| IV | Any T, Any N, M1 | |
| IVA | Any T, Any N, M1a | 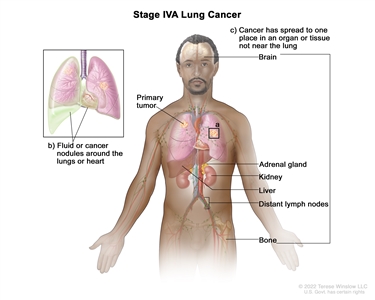 |
| Any T, Any N, M1b | ||
| IVB | Any T, Any N, M1c | 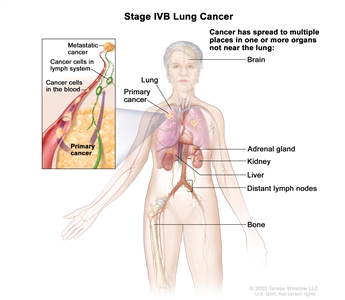 |
References:
- Pfister DG, Johnson DH, Azzoli CG, et al.: American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 22 (2): 330-53, 2004.
- Ludwig MS, Goodman M, Miller DL, et al.: Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 128 (3): 1545-50, 2005.
- Webb WR, Gatsonis C, Zerhouni EA, et al.: CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology 178 (3): 705-13, 1991.
- Toloza EM, Harpole L, McCrory DC: Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 123 (1 Suppl): 137S-146S, 2003.
- Gould MK, Kuschner WG, Rydzak CE, et al.: Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 139 (11): 879-92, 2003.
- Dwamena BA, Sonnad SS, Angobaldo JO, et al.: Metastases from non-small cell lung cancer: mediastinal staging in the 1990s--meta-analytic comparison of PET and CT. Radiology 213 (2): 530-6, 1999.
- Ung YC, Maziak DE, Vanderveen JA, et al.: 18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a systematic review. J Natl Cancer Inst 99 (23): 1753-67, 2007.
- Dietlein M, Weber K, Gandjour A, et al.: Cost-effectiveness of FDG-PET for the management of potentially operable non-small cell lung cancer: priority for a PET-based strategy after nodal-negative CT results. Eur J Nucl Med 27 (11): 1598-609, 2000.
- Scott WJ, Shepherd J, Gambhir SS: Cost-effectiveness of FDG-PET for staging non-small cell lung cancer: a decision analysis. Ann Thorac Surg 66 (6): 1876-83; discussion 1883-5, 1998.
- Gambhir SS, Hoh CK, Phelps ME, et al.: Decision tree sensitivity analysis for cost-effectiveness of FDG-PET in the staging and management of non-small-cell lung carcinoma. J Nucl Med 37 (9): 1428-36, 1996.
- van Tinteren H, Hoekstra OS, Smit EF, et al.: Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 359 (9315): 1388-93, 2002.
- Viney RC, Boyer MJ, King MT, et al.: Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small-cell lung cancer. J Clin Oncol 22 (12): 2357-62, 2004.
- Vansteenkiste JF, Stroobants SG, De Leyn PR, et al.: Lymph node staging in non-small-cell lung cancer with FDG-PET scan: a prospective study on 690 lymph node stations from 68 patients. J Clin Oncol 16 (6): 2142-9, 1998.
- Roberts PF, Follette DM, von Haag D, et al.: Factors associated with false-positive staging of lung cancer by positron emission tomography. Ann Thorac Surg 70 (4): 1154-9; discussion 1159-60, 2000.
- Liewald F, Grosse S, Storck M, et al.: How useful is positron emission tomography for lymphnode staging in non-small-cell lung cancer? Thorac Cardiovasc Surg 48 (2): 93-6, 2000.
- Yokoi K, Kamiya N, Matsuguma H, et al.: Detection of brain metastasis in potentially operable non-small cell lung cancer: a comparison of CT and MRI. Chest 115 (3): 714-9, 1999.
- Mountain CF: Revisions in the International System for Staging Lung Cancer. Chest 111 (6): 1710-7, 1997.
- Lung. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 431–56.
Treatment Option Overview for NSCLC
In non-small cell lung cancer (NSCLC), results of standard treatment are poor except for the most localized cancers. All newly diagnosed patients with NSCLC are potential candidates for studies evaluating new forms of treatment.
Treatment decisions are based on some of the following factors:
- Knowledge of histological type and molecular features.
- Tumor size and location.
- Involvement of pleura.
- Surgical margins.
- Status and location of lymph nodes by station.
- Tumor grade.
- Lymphovascular invasion.
Surgery is potentially the most curative therapeutic option for this disease. Postoperative chemotherapy may provide an additional benefit to patients with resected NSCLC. Radiation therapy combined with chemotherapy can produce a cure in a small number of patients and can provide palliation in most patients. Prophylactic cranial irradiation may reduce the incidence of brain metastases, but there is no evidence of a survival benefit and the effect of prophylactic cranial irradiation on quality of life is not known.[1,2] In patients with advanced-stage disease, chemotherapy or epidermal growth factor receptor (EGFR) kinase inhibitors offer modest improvements in median survival, although overall survival is poor.[3,4]
Chemotherapy has produced short-term improvement in disease-related symptoms in patients with advanced NSCLC. Several clinical trials have attempted to assess the impact of chemotherapy on tumor-related symptoms and quality of life. In total, these studies suggest that tumor-related symptoms may be controlled by chemotherapy without adversely affecting overall quality of life;[5,6] however, the impact of chemotherapy on quality of life requires more study. In general, medically eligible older patients with good performance status obtain the same benefits from treatment as younger patients.
The identification of gene mutations in lung cancer has led to the development of molecularly targeted therapy to improve the survival of subsets of patients with metastatic disease.[7] In particular, genetic abnormalities in EGFR, MAPK, and PI3K signaling pathways in subsets of NSCLC may define mechanisms of drug sensitivity and primary or acquired resistance to kinase inhibitors. EGFR mutations strongly predict the improved response rate and progression-free survival of inhibitors of EGFR. Fusions of ALK with EML4 and other genes form translocation products that occur in ranges from 3% to 7% in unselected NSCLC and are responsive to pharmacological inhibition of ALK by agents such as alectinib. The MET oncogene encodes hepatocyte growth factor receptor. Amplification of this gene has been associated with secondary resistance to EGFR tyrosine kinase inhibitors. Recurrent fusions involving the ROS1 gene are observed in up to 2% of NSCLCs and are responsive to treatment with crizotinib and entrectinib. NTRK gene fusions can occur in up to 1% of NSCLCs and can be treated with the TRK inhibitors, larotrectinib and entrectinib. For more information, see the Molecular Features section.
The treatment options for each stage of NSCLC are presented in Table 5.
| Stage (TNM Definitions) | Treatment Options | |
|---|---|---|
| ALK = anaplastic lymphoma kinase; EGFR = epidermal growth factor receptor; HER2 = human epidermal growth factor receptor 2; mTOR = mammalian target of rapamycin; NSCLC = non-small cell lung cancer; NTRK = neurotrophic tyrosine kinase; TKIs = tyrosine kinase inhibitors; TNM = tumor, node, metastasis. | ||
| Occult NSCLC | Surgery | |
| Stage 0 NSCLC | Surgery | |
| Endobronchial therapies | ||
| Stages IA and IB NSCLC | Surgery | |
| Adjuvant therapy | ||
| Radiation therapy | ||
| Stages IIA and IIB NSCLC | Surgery with or without adjuvant and/or neoadjuvant therapy | |
| Radiation therapy | ||
| Stage IIIA NSCLC | Resected or resectable disease | Surgery with neoadjuvant and/or adjuvant therapy |
| Neoadjuvant therapy | ||
| Perioperative (neoadjuvant and adjuvant) immunotherapy with chemotherapy | ||
| Adjuvant therapy | ||
| Unresectable disease | Chemoradiation therapy | |
| Radiation therapy | ||
| Superior sulcus tumors | Surgery | |
| Chemoradiation therapy followed by surgery | ||
| Radiation therapy alone | ||
| Tumors that invade the chest wall | Surgery | |
| Surgery and radiation therapy | ||
| Radiation therapy alone | ||
| Chemotherapy combined with radiation therapy and/or surgery | ||
| Stages IIIB and IIIC NSCLC | Sequential or concurrent chemotherapy and radiation therapy | |
| Radiation therapy alone | ||
| Newly Diagnosed Stage IV, Relapsed, and Recurrent NSCLC | Cytotoxic combination chemotherapy | |
| Combination chemotherapy with monoclonal antibodies | ||
| Maintenance therapy after first-line chemotherapy(for patients with stable or responding disease after four cycles of platinum-based combination chemotherapy) | ||
| EGFR TKIs with or without chemotherapy(for patients withEGFRmutations) | ||
| EGFR-directed therapy(for patients withEGFRexon 20 insertion mutations) | ||
| ALK inhibitors(for patients withALKtranslocations) | ||
| BRAF V600E and MEK inhibitors(for patients withBRAFV600E mutations) | ||
| ROS1 inhibitors(for patients withROS1rearrangements) | ||
| NTRK inhibitors(for patients withNTRKfusions) | ||
| RET inhibitors(for patients withRETfusions) | ||
| MET inhibitors(for patients withMETexon 14 skipping mutations) | ||
| Immune checkpoint inhibitors with or without chemotherapy | ||
| mTOR inhibitors(for patients with unresectable, locally advanced or metastatic, progressive, well-differentiated, nonfunctional, neuroendocrine tumors) | ||
| Local therapies and special considerations | ||
| Progressive Stage IV, Relapsed, and Recurrent NSCLC | Chemotherapy | |
| EGFR-directed therapy | ||
| ALK-directed TKIs | ||
| BRAF V600E and MEK inhibitors(for patients withBRAFV600E mutations) | ||
| ROS1-directed therapy | ||
| NTRK inhibitors(for patients withNTRKfusions) | ||
| RET inhibitors(for patients withRETfusions) | ||
| MET inhibitors(for patients withMETexon 14 skipping mutations) | ||
| KRAS G12C inhibitors(for patients withKRASG12C mutations) | ||
| HER2-targeted therapy(for patients withHER2mutations) | ||
| Immunotherapy | ||
| mTOR inhibitors(for patients with unresectable, locally advanced or metastatic, progressive, well-differentiated, nonfunctional, neuroendocrine tumors) | ||
In addition to the treatment options presented in Table 5, treatment options under clinical evaluation include:
- Combining local treatment (surgery).
- Regional treatment (radiation therapy).
- Systemic treatments (chemotherapy, immunotherapy, and targeted agents).
- Developing more effective systemic therapy.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Lester JF, MacBeth FR, Coles B: Prophylactic cranial irradiation for preventing brain metastases in patients undergoing radical treatment for non-small-cell lung cancer: a Cochrane Review. Int J Radiat Oncol Biol Phys 63 (3): 690-4, 2005.
- Pöttgen C, Eberhardt W, Grannass A, et al.: Prophylactic cranial irradiation in operable stage IIIA non small-cell lung cancer treated with neoadjuvant chemoradiotherapy: results from a German multicenter randomized trial. J Clin Oncol 25 (31): 4987-92, 2007.
- Chemotherapy for non-small cell lung cancer. Non-small Cell Lung Cancer Collaborative Group. Cochrane Database Syst Rev (2): CD002139, 2000.
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 311 (7010): 899-909, 1995.
- Spiro SG, Rudd RM, Souhami RL, et al.: Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax 59 (10): 828-36, 2004.
- Clegg A, Scott DA, Hewitson P, et al.: Clinical and cost effectiveness of paclitaxel, docetaxel, gemcitabine, and vinorelbine in non-small cell lung cancer: a systematic review. Thorax 57 (1): 20-8, 2002.
- Pao W, Girard N: New driver mutations in non-small-cell lung cancer. Lancet Oncol 12 (2): 175-80, 2011.
Treatment of Occult NSCLC
In occult lung cancer, a diagnostic evaluation often includes chest x-ray and selective bronchoscopy with close follow-up (e.g., computed tomography scan), when needed, to define the site and nature of the primary tumor; tumors discovered in this fashion are generally early stage and curable by surgery.
After discovery of the primary tumor, treatment involves establishing the stage of the tumor. Therapy is identical to that recommended for other non-small cell lung cancer (NSCLC) patients with similar-stage disease.
Treatment Options for Occult NSCLC
Treatment options for occult NSCLC include:
- Surgery.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Treatment of Stage 0 NSCLC
Stage 0 non-small cell lung cancer (NSCLC) frequently progresses to invasive cancer.[1,2,3] Patients may be offered surveillance bronchoscopies and, if lesions are detected, potentially curative therapies.
Treatment Options for Stage 0 NSCLC
Treatment options for stage 0 NSCLC include:
- Surgery.
- Endobronchial therapies, including photodynamic therapy, electrocautery, cryotherapy, and neodymium-doped yttrium aluminum garnet (Nd-YAG) laser therapy.
Surgery
Segmentectomy or wedge resection are used to preserve maximum normal pulmonary tissue because patients with stage 0 NSCLC are at a high risk of second lung cancers. Because these tumors are by definition noninvasive and incapable of metastasizing, they should be curable with surgical resection; however, such lesions, when identified, are often centrally located and may require a lobectomy.
Endobronchial therapies
Patients with central lesions may be candidates for curative endobronchial therapy. Endobronchial therapies that preserve lung function include photodynamic therapy, electrocautery, cryotherapy, and Nd-YAG laser therapy.[3,4,5,6]
Evidence (endobronchial therapies):
- Small case series have reported high complete response rates and long-term survival in selected patients.[7,8][Level of evidence C2]
Efficacy of these treatment modalities in the management of patients with early NSCLC remains to be proven in definitive randomized controlled trials.
A high incidence of second primary cancers develop in these patients.[1,2]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Woolner LB, Fontana RS, Cortese DA, et al.: Roentgenographically occult lung cancer: pathologic findings and frequency of multicentricity during a 10-year period. Mayo Clin Proc 59 (7): 453-66, 1984.
- Venmans BJ, van Boxem TJ, Smit EF, et al.: Outcome of bronchial carcinoma in situ. Chest 117 (6): 1572-6, 2000.
- Jeremy George P, Banerjee AK, Read CA, et al.: Surveillance for the detection of early lung cancer in patients with bronchial dysplasia. Thorax 62 (1): 43-50, 2007.
- Kennedy TC, McWilliams A, Edell E, et al.: Bronchial intraepithelial neoplasia/early central airways lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132 (3 Suppl): 221S-233S, 2007.
- Corti L, Toniolo L, Boso C, et al.: Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med 39 (5): 394-402, 2007.
- Deygas N, Froudarakis M, Ozenne G, et al.: Cryotherapy in early superficial bronchogenic carcinoma. Chest 120 (1): 26-31, 2001.
- van Boxem TJ, Venmans BJ, Schramel FM, et al.: Radiographically occult lung cancer treated with fibreoptic bronchoscopic electrocautery: a pilot study of a simple and inexpensive technique. Eur Respir J 11 (1): 169-72, 1998.
- van Boxem AJ, Westerga J, Venmans BJ, et al.: Photodynamic therapy, Nd-YAG laser and electrocautery for treating early-stage intraluminal cancer: which to choose? Lung Cancer 31 (1): 31-6, 2001.
Treatment of Stages IA and IB NSCLC
Treatment Options for Stages IA and IB NSCLC
Treatment options for stages IA non-small cell lung cancer (NSCLC) and IB NSCLC include:
- Surgery.
- Adjuvant therapy.
- Adjuvant chemotherapy (for patients with large stage IB tumors).
- Adjuvant targeted therapy (for patients with stage IB tumors with EGFR mutations).
- Adjuvant immunotherapy (for patients with stage IB tumors >4 cm).
- Radiation therapy (for patients who cannot have surgery or choose not to have surgery).
Chemotherapy and radiation therapy have not been shown to improve survival in patients with stage I NSCLC that has been completely resected.
Surgery
Surgery is the treatment of choice for patients with stage I NSCLC. A lobectomy or segmental, wedge, or sleeve resection may be performed as appropriate. Patients with impaired pulmonary function are candidates for segmental or wedge resection of the primary tumor. Careful preoperative assessment of the patient's overall medical condition, especially the patient's pulmonary reserve, is critical in considering the benefits of surgery. The immediate postoperative mortality rate is age related, but a 3% to 5% mortality rate with lobectomy can be expected.[1]
Evidence (surgery):
- The Lung Cancer Study Group conducted a randomized study (LCSG-821) that compared lobectomy with limited resection for patients with stage I lung cancer. Results of the study showed:[2]
- A reduction in local recurrence for patients treated with lobectomy compared with those treated with limited excision.
- No significant difference in overall survival (OS).
- Similar results were reported from a nonrandomized comparison of anatomic segmentectomy and lobectomy.[3]
- A survival advantage was noted with lobectomy for patients with tumors larger than 3 cm but not for those with tumors smaller than 3 cm.
- The rate of locoregional recurrence was significantly less after lobectomy, regardless of primary tumor size.
- A study of stage I patients showed:[4]
- Those treated with wedge or segmental resections had a local recurrence rate of 50% (i.e., 31 recurrences out of 62 patients) despite having undergone complete resections.[4]
- A multicenter, noninferiority, phase III trial (NCT00499330) evaluated lobar or sublobar resection in patients with peripheral stage IA NSCLC. A total of 697 patients with clinical stage T1a, N0 tumors (tumor size <2 cm) were randomly assigned to undergo sublobar resection or lobar resection after intraoperative confirmation of node-negative disease in the hilar and mediastinal lymph nodes. The primary end point was disease-free survival (DFS).[5][Level of evidence B1]
- After a median follow-up of 7 years, sublobar resection was noninferior to lobar resection for DFS (hazard ratio [HR], 1.01; 90% confidence interval [CI], 0.83–1.24; one-sided P = .02 for noninferiority).
- OS was similar after sublobar resection or lobar resection (HR, 0.95; 95% CI, 0.72–1.26).
- No substantial differences were noted in the incidence of locoregional or distant disease recurrence between the two groups.
- At 6 months after surgery, the magnitude of reduction from baseline in the percentage of predicted FEV1 (forced expiratory volume in first second of expiration) was greater in the lobectomy group (-6%; 95% CI, -8% to -5%) versus the sublobar resection group (-4%; 95% CI, -5% to -2%). The magnitude of reduction in the percentage of predicted FVC (forced vital capacity) was also greater after lobectomy (-5%; 95% CI, -7% to -3%) than after sublobar resection (-3%; 95% CI, -4% to -1%).
These results suggest that sublobar resection by anatomical segmentectomy or wedge resection is effective for management of clinical stage T1a, N0 NSCLC when intraoperative sampling of hilar and mediastinal lymph nodes is negative.
- The Cochrane Collaboration reviewed 11 randomized trials with a total of 1,910 patients who underwent surgical interventions for early-stage (I–IIIA) lung cancer.[6] A pooled analysis of three trials reported the following:
- Four-year survival was superior in patients with resectable stage I, II, or IIIA NSCLC who underwent resection and complete ipsilateral mediastinal lymph node dissection (CMLND), compared with those who underwent resection and lymph node sampling; the HR was estimated to be 0.78 (95% CI, 0.65–0.93, P = .005).[6][Level of evidence A1]
- There was a significant reduction in any cancer recurrence (local or distant) in the CMLND group (relative risk [RR], 0.79; 95% CI, 0.66–0.95; P = .01) that appeared mainly because of a reduction in the number of distant recurrences (RR, 0.78; 95% CI, 0.61–1.00; P = .05).
- There was no difference in operative mortality.
- Air leak lasting more than 5 days was significantly more common in patients assigned to CMLND (RR, 2.94; 95% CI, 1.01–8.54; P = .05).
- CMLND versus lymph node sampling was evaluated in a large, randomized, phase III trial (ACOSOG-Z0030 [NCT00003831]).[7,8]
Current evidence suggests that lung cancer resection combined with CMLND is not associated with improvement in survival compared with lung cancer resection combined with systematic sampling of mediastinal lymph nodes in patients with stage I, II, or IIIA NSCLC.[8][Level of evidence A1]
Conclusions about the efficacy of surgery for patients with local and locoregional NSCLC are limited by the small number of participants studied to date and the potential methodological weaknesses of the trials.
Adjuvant therapy
Many patients who have surgery subsequently develop regional or distant metastases.[9] Such patients are candidates for entry into clinical trials evaluating postoperative treatment with chemotherapy or radiation therapy following surgery. At present, neither chemotherapy nor radiation therapy has been found to improve survival in patients with stage I NSCLC that has been completely resected.
Adjuvant chemotherapy
Based on a meta-analysis, postoperative chemotherapy is not recommended outside of a clinical trial for patients with completely resected stage I NSCLC.[10] However, there may be some benefit of adjuvant chemotherapy in patients with stage IB tumors that are larger than 4 cm.
Evidence (adjuvant chemotherapy for patients with stage IB NSCLC):
- The Cancer and Leukemia Group B study (CALGB-9633 [NCT00002852]) addressed the results of adjuvant carboplatin and paclitaxel versus observation for OS in 344 patients with resected stage IB (i.e., pathological T2, N0) NSCLC. Within 4 to 8 weeks of resection, patients were randomly assigned to postoperative chemotherapy or observation.[11]
- Survival was not significantly different (HR, 0.83; 90% CI, 0.64–1.08; P = .12) at a median follow-up of 74 months.
- Grades 3 to 4 neutropenia were the predominant toxicity; there were no treatment-related deaths.
- A post-hoc exploratory analysis demonstrated a significant survival difference in favor of postoperative chemotherapy for patients who had tumors 4 cm or larger in diameter (HR, 0.69; 90% CI, 0.48–0.99; P = .043).
Given the magnitude of observed survival differences, CALGB-9633 may have been underpowered to detect small but clinically meaningful improvements in survival. In addition, the use of a carboplatin versus a cisplatin combination might have affected the results. At present, there is no reliable evidence that postoperative chemotherapy improves survival of patients with stage IB NSCLC.[11][Level of evidence A1]
Adjuvant targeted therapy (for patients with stage IB NSCLC withEGFRmutations)
Adjuvant targeted therapy with osimertinib for patients with EGFR-mutated NSCLC and resected stage IB to IIIA NSCLC was studied in a phase III clinical trial and showed improved OS.
Evidence (adjuvant targeted therapy with osimertinib for patients with stage IB EGFR-mutated NSCLC):
- The ADAURA (NCT02511106) phase III, double-blind, placebo-controlled trial randomly assigned 682 patients with surgically resected stage IB to stage IIIA NSCLC with EGFR-sensitizing mutations (centrally determined, deletion in exon 19 or L858R mutation in exon 21) to receive either osimertinib 80 mg by mouth daily or a placebo for 3 years. Standard postoperative adjuvant chemotherapy was allowed but not mandatory; decisions regarding adjuvant chemotherapy were made by the physician and patient before trial enrollment. There were 399 patients who received osimertinib and 342 patients who received placebo.[12][Level of evidence A1]
- In the overall population, the 5-year OS rate was 88% in the osimertinib group and 78% in the placebo group (overall HRdeath, 0.49; 95.03% CI, 0.34–0.70; P < .001).
- Among patients with stage II to IIIA disease, the 5-year OS rate was 85% in the osimertinib group and 73% in the placebo group (overall HRdeath, 0.49; 95.03% CI, 0.33–0.73; P < .001).
- The adverse event profile is consistent with other studies that used osimertinib except for pneumonia related to COVID-19, which was reported later.
The U.S. Food and Drug Administration (FDA) approved osimertinib as adjuvant therapy for patients with stage IB to IIIA NSCLC with EGFR exon 19 deletions or exon 21 L858R mutations.
Adjuvant immunotherapy
Evidence (adjuvant immunotherapy with pembrolizumab for patients with stage IB tumors >4 cm):
- The phase III, multicenter, open-label PEARLS/KEYNOTE-091 trial (NCT02504372) randomly assigned 1,177 patients with completely resected stage IB (tumor >4 cm) to stage IIIA NSCLC to receive pembrolizumab (200 mg every 3 weeks) or placebo for up to 18 cycles, or until disease progression or unacceptable toxicity. Patients started study treatment after resection or, if indicated, after adjuvant chemotherapy (maximum of four cycles). The dual primary end points were DFS in the overall study population and DFS in patients with a programmed death-ligand 1 (PD-L1) tumor proportion score (TPS) of 50% or greater, as determined using the PD-L1 IHC 22C3 pharmDx assay. These end points were reported in a prespecified interim analysis after a median follow-up of 35.6 months (interquartile range, 27.1–45.5).[13][Level of evidence B1]
- In the overall study population, the median DFS was 53.6 months (95% CI, 39.2–not reached [NR]) in the pembrolizumab group and 42.0 months (95% CI, 31.3–NR) in the placebo group (HR, 0.76; 95% CI, 0.63–0.91; P = .0014).
- In the PD-L1 TPS ≥50% population, the median DFS was not reached with either pembrolizumab (95% CI, 44.3–NR) or placebo (95% CI, 35.8–NR) (HR, 0.82; 95% CI, 0.57–1.18; P = .14).
- OS data were immature at the time of the prespecified interim analysis.
- No new safety signals were identified in this study.
The FDA approved pembrolizumab as a single agent for adjuvant treatment following resection and platinum-based chemotherapy for patients with stage IB (T2a ≥4 cm), II, or IIIA NSCLC. Of note, the FDA label specifies that pembrolizumab can be used as adjuvant therapy after platinum-based chemotherapy. However, chemotherapy was not required in the overall study patient population evaluated in KEYNOTE-091.
Adjuvant external radiation therapy
The value of postoperative (adjuvant) radiation therapy (PORT) has been evaluated and has not been found to improve the outcome of patients with completely resected stage I NSCLC.[14]
Adjuvant brachytherapy
The value of intraoperative (adjuvant) brachytherapy applied to the suture line has been evaluated in patients undergoing sublobar resections for stage I NSCLC to improve local control; it has not been found to improve outcomes.
Evidence (adjuvant brachytherapy):
- A phase III trial that randomly assigned 222 patients to undergo sublobar resection with or without suture line brachytherapy reported the following:[15]
- No difference in the primary end point of local recurrence (5-year estimate, 14.0% vs. 16.7%; P = .59).
- No difference in OS rates (5-year estimate, 61.4% vs. 55.6%; P = .38).[15][Level of evidence B1] vs. [Level of evidence A1]
Radiation therapy
A substantial number of patients are ineligible for standard surgical resection because of comorbid conditions that are associated with unacceptably high perioperative risk. Patients with potentially resectable tumors with medical contraindications to surgery or those with inoperable stage I disease and with sufficient pulmonary reserve may be candidates for radiation therapy with curative intent.[16,17,18] Nonrandomized observational studies comparing treatment outcomes associated with resection, radiation therapy, and observation have demonstrated shorter survival times and higher mortality for patients who undergo observation only.[16,19]
Conventional radiation therapy
Historically, conventional primary radiation therapy consisted of approximately 60 Gy to 70 Gy delivered with megavoltage equipment to the midplane of the known tumor volume using conventional fractionation (1.8–2.0 Gy per day).
Improvements in radiation techniques include planning techniques to account for tumor motion, more conformal planning techniques (e.g., 3-D conformal radiation therapy and intensity-modulated radiation therapy), and image guidance during treatment. Modern approaches to delivery of external-beam radiation therapy (EBRT) include hypofractionated radiation therapy and stereotactic body radiation therapy (SBRT). However, there are limited reliable data from comparative trials to determine which approaches yield superior outcomes.[17,18]
Evidence (conventional radiation therapy):
- In the largest retrospective conventional radiation therapy series, patients with inoperable disease were treated with definitive radiation therapy.[20,21,22]
- Patients achieved 5-year survival rates of 10% to 30%.[20,21,22]
- Several series demonstrated that patients with T1, N0 tumors had better outcomes, and 5-year survival rates of 30% to 60% were found in this subgroup.[20,21,23]
- However, local-only failure occurs in as many as 50% of patients treated with conventional radiation therapy to doses in the range of 60 Gy to 65 Gy.[24,25]
- A single report of patients older than 70 years who had resectable lesions smaller than 4 cm but who had medically inoperable disease or who refused surgery reported the following:[23]
- Survival at 5 years after radiation therapy with curative intent was comparable with a historical control group of patients of similar age who were resected with curative intent.
- A small case series using matched controls reported the following:[4]
- The addition of endobronchial brachytherapy improved local disease control compared with EBRT.[4][Level of evidence C2]
Hypofractionated radiation therapy
Hypofractionated radiation therapy involves the delivery of a slightly higher dose of radiation therapy per day (e.g., 2.4–4.0 Gy) over a shorter period of time compared with conventionally fractionated radiation therapy. Multiple prospective phase I/II trials have demonstrated that hypofractionated radiation therapy to a dose of 60 Gy to 70 Gy delivered over 3 to 4 weeks with 2.4 Gy to 4.0 Gy per day resulted in a low incidence of moderate to severe toxicity, 2-year OS rates of 50% to 60%, and 2-year tumor local control of 80% to 90%.[26,27,28][Level of evidence C1]
Stereotactic body radiation therapy (SBRT)
SBRT involves the delivery of highly conformal, high-dose radiation therapy over an extremely hypofractionated course (e.g., one to five treatments) delivered over 1 to 2 weeks. Commonly used regimens include 18 Gy × 3, 12 Gy to 12.5 Gy × 4, and 10 Gy to 12 Gy × 5, and deliver a substantially higher biologically effective dose compared with historic conventional radiation therapy regimens.
Multiple prospective phase I/II trials and institutional series have demonstrated that SBRT results in a low incidence of pulmonary toxicity (<10% risk of symptomatic radiation pneumonitis), 2-year OS rates of 50% to 60%, and 2-year tumor control of 90% to 95%.[29,30,31,32,33,34,35][Level of evidence C1]
Evidence (SBRT):
- Early phase I/II trials from Indiana University identified the maximum tolerated dose of three-fraction SBRT at 18 Gy × 3 for T1 tumors.
- This regimen resulted in a 2-year OS rate of 55% and 2-year local tumor control of 95%.
- An unacceptably high incidence (8.6%) of grade 5 toxicity was observed in patients with central tumors (defined as within 2 cm of the tracheobronchial tree from the trachea to the level of the lobar bronchi).[30]
- A subsequent multicenter trial (RTOG-0236 [NCT00087438]) studied the 18 Gy × 3 regimen in 55 patients with peripheral T1 to T2 tumors only.
- This trial demonstrated a 3-year OS rate of 56% and 3-year primary tumor control of 98%.
- The incidence of moderate to severe toxicity was low, with grade 3 toxicity in 24% of patients, grade 4 toxicity in 4% of patients, and no grade 5 toxicity, with a 4% incidence of grade 3 radiation pneumonitis.[34]
- In the largest reported series from VU University Medical Center Amsterdam, 676 patients with T1 to T2 tumors were treated with three-, five-, and eight-fraction SBRT using a risk-adapted approach (a tailored fractionation regimen based on tumor proximity to critical organs).
- With a median follow-up of 32.9 months, the median OS was 40.7 months, and 2-year local tumor control was 95%.[35]
- While central location is a contraindication to three-fraction SBRT based on data from the Indiana phase II study, a subsequent systematic review of published reports of 315 patients with 563 central tumors demonstrated a much lower incidence of severe toxicity, including a 1% to 5% risk of grade 5 events with more protracted SBRT regimens (e.g., four to ten fractions).[36]
- A multicenter phase I/II trial (RTOG-0813 [NCT00750269]) is ongoing to identify the maximum tolerated dose for a five-fraction SBRT regimen for central tumors.
A randomized trial of hypofractionated radiation therapy versus SBRT (LUSTRE [NCT01968941]) is ongoing to determine the optimal radiation therapy regimen, but SBRT has been widely adopted for patients with medically inoperable stage I NSCLC.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Ginsberg RJ, Hill LD, Eagan RT, et al.: Modern thirty-day operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg 86 (5): 654-8, 1983.
- Ginsberg RJ, Rubinstein LV: Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 60 (3): 615-22; discussion 622-3, 1995.
- Warren WH, Faber LP: Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 107 (4): 1087-93; discussion 1093-4, 1994.
- Mantz CA, Dosoretz DE, Rubenstein JH, et al.: Endobronchial brachytherapy and optimization of local disease control in medically inoperable non-small cell lung carcinoma: a matched-pair analysis. Brachytherapy 3 (4): 183-90, 2004.
- Altorki N, Wang X, Kozono D, et al.: Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 388 (6): 489-498, 2023.
- Manser R, Wright G, Hart D, et al.: Surgery for early stage non-small cell lung cancer. Cochrane Database Syst Rev (1): CD004699, 2005.
- Allen MS, Darling GE, Pechet TT, et al.: Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 81 (3): 1013-9; discussion 1019-20, 2006.
- Darling GE, Allen MS, Decker PA, et al.: Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 141 (3): 662-70, 2011.
- Martini N, Bains MS, Burt ME, et al.: Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 109 (1): 120-9, 1995.
- Pignon JP, Tribodet H, Scagliotti GV, et al.: Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 26 (21): 3552-9, 2008.
- Strauss GM, Herndon JE, Maddaus MA, et al.: Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 26 (31): 5043-51, 2008.
- Tsuboi M, Herbst RS, John T, et al.: Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N Engl J Med 389 (2): 137-147, 2023.
- O'Brien M, Paz-Ares L, Marreaud S, et al.: Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 23 (10): 1274-1286, 2022.
- PORT Meta-analysis Trialists Group: Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev (2): CD002142, 2005.
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al.: Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small-cell lung cancer. J Clin Oncol 32 (23): 2456-62, 2014.
- McGarry RC, Song G, des Rosiers P, et al.: Observation-only management of early stage, medically inoperable lung cancer: poor outcome. Chest 121 (4): 1155-8, 2002.
- Lanni TB, Grills IS, Kestin LL, et al.: Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. Am J Clin Oncol 34 (5): 494-8, 2011.
- Grutters JP, Kessels AG, Pijls-Johannesma M, et al.: Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother Oncol 95 (1): 32-40, 2010.
- Raz DJ, Zell JA, Ou SH, et al.: Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 132 (1): 193-9, 2007.
- Dosoretz DE, Katin MJ, Blitzer PH, et al.: Radiation therapy in the management of medically inoperable carcinoma of the lung: results and implications for future treatment strategies. Int J Radiat Oncol Biol Phys 24 (1): 3-9, 1992.
- Gauden S, Ramsay J, Tripcony L: The curative treatment by radiotherapy alone of stage I non-small cell carcinoma of the lung. Chest 108 (5): 1278-82, 1995.
- Sibley GS, Jamieson TA, Marks LB, et al.: Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys 40 (1): 149-54, 1998.
- Noordijk EM, vd Poest Clement E, Hermans J, et al.: Radiotherapy as an alternative to surgery in elderly patients with resectable lung cancer. Radiother Oncol 13 (2): 83-9, 1988.
- Dosoretz DE, Galmarini D, Rubenstein JH, et al.: Local control in medically inoperable lung cancer: an analysis of its importance in outcome and factors determining the probability of tumor eradication. Int J Radiat Oncol Biol Phys 27 (3): 507-16, 1993.
- Kaskowitz L, Graham MV, Emami B, et al.: Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 27 (3): 517-23, 1993.
- Bradley J, Graham MV, Winter K, et al.: Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 61 (2): 318-28, 2005.
- Bogart JA, Hodgson L, Seagren SL, et al.: Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol 28 (2): 202-6, 2010.
- Cheung P, Faria S, Ahmed S, et al.: Phase II study of accelerated hypofractionated three-dimensional conformal radiotherapy for stage T1-3 N0 M0 non-small cell lung cancer: NCIC CTG BR.25. J Natl Cancer Inst 106 (8): , 2014.
- Timmerman R, Papiez L, McGarry R, et al.: Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 124 (5): 1946-55, 2003.
- Timmerman R, McGarry R, Yiannoutsos C, et al.: Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 24 (30): 4833-9, 2006.
- Lagerwaard FJ, Haasbeek CJ, Smit EF, et al.: Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 70 (3): 685-92, 2008.
- Baumann P, Nyman J, Hoyer M, et al.: Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 27 (20): 3290-6, 2009.
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al.: Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 75 (3): 677-82, 2009.
- Timmerman R, Paulus R, Galvin J, et al.: Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303 (11): 1070-6, 2010.
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al.: Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 13 (8): 802-9, 2012.
- Senthi S, Haasbeek CJ, Slotman BJ, et al.: Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 106 (3): 276-82, 2013.
Treatment of Stages IIA and IIB NSCLC
Treatment Options for Stages IIA and IIB NSCLC
Treatment options for stages IIA non-small cell lung cancer (NSCLC) and IIB NSCLC include:
- Surgery with or without adjuvant and/or neoadjuvant therapy.
- Surgery alone.
- Adjuvant chemotherapy.
- Adjuvant targeted therapy (for patients with EGFR mutations).
- Adjuvant immunotherapy.
- Adjuvant radiation therapy.
- Neoadjuvant chemotherapy.
- Neoadjuvant immunotherapy with chemotherapy.
- Perioperative (neoadjuvant and adjuvant) immunotherapy with neoadjuvant chemotherapy.
- Radiation therapy (for patients who cannot have surgery).
- Clinical trials of radiation therapy after curative surgery.
Adjuvant radiation therapy has not been shown to improve outcomes in patients with stage II NSCLC.
Surgery with or without adjuvant or neoadjuvant therapy
Surgery alone
Surgery is the treatment of choice for patients with stage II NSCLC. A lobectomy, pneumonectomy, segmental resection, wedge resection, or sleeve resection may be performed as appropriate. Careful preoperative assessment of the patient's overall medical condition, especially the patient's pulmonary reserve, is critical in considering the benefits of surgery. In addition to the immediate and age-related postoperative mortality rate, a 5% to 8% mortality rate with pneumonectomy or a 3% to 5% mortality rate with lobectomy can be expected.
Evidence (surgery):
- The Cochrane Collaboration reviewed 11 randomized trials with a total of 1,910 patients who underwent surgical interventions for early-stage (I–IIIA) lung cancer.[1] A pooled analysis of three trials reported the following:
- Four-year survival was superior in patients with resectable stage I, II, or IIIA NSCLC who underwent resection and complete ipsilateral mediastinal lymph node dissection (CMLND), compared with those who underwent resection and lymph node sampling; the hazard ratio (HR) was estimated to be 0.78 (95% confidence interval [CI], 0.65–0.93; P = .005).[1][Level of evidence A1]
- There was a significant reduction in any cancer recurrence (local or distant) in the CMLND group (relative risk [RR], 0.79; 95% CI, 0.66–0.95; P = .01) that appeared mainly as the result of a reduction in the number of distant recurrences (RR, 0.78; 95% CI, 0.61–1.00; P = .05).
- There was no difference in operative mortality.
- Air leak lasting more than 5 days was significantly more common in patients assigned to CMLND (RR, 2.94; 95% CI, 1.01–8.54; P = .05).
- CMLND versus lymph node sampling was evaluated in a large randomized phase III trial (ACOSOG-Z0030 [NCT00003831]).[2]
Evidence suggests that lung cancer resection combined with CMLND is not associated with improvement in survival compared with lung cancer resection combined with systematic sampling of mediastinal lymph nodes in patients with stage I, II, or IIIA NSCLC.[3][Level of evidence A1]
Conclusions about the efficacy of surgery for patients with local and locoregional NSCLC are limited by the small number of participants studied and potential methodological weaknesses of the trials.
Adjuvant chemotherapy
The preponderance of evidence indicates that postoperative cisplatin combination chemotherapy provides a significant survival advantage to patients with resected stage II NSCLC. Preoperative chemotherapy may also provide survival benefit. The optimal sequence of surgery and chemotherapy and the benefits and risks of postoperative radiation therapy in patients with resectable NSCLC remain to be determined.
After surgery, many patients develop regional or distant metastases.[4] Several randomized controlled trials and meta-analyses have evaluated the use of postoperative chemotherapy in patients with stage I, II, and IIIA NSCLC.[5,6,7,8,9,10,11]
Evidence (adjuvant chemotherapy):
- Data on individual patient outcomes were collected and pooled into a meta-analysis from the five largest trials (4,584 patients) of cisplatin-based chemotherapy in patients with completely resected NSCLC that were conducted after 1995.[7]
- With a median follow-up time of 5.2 years, the overall HRdeath was 0.89 (95% CI, 0.82–0.96; P = .005), corresponding to a 5-year absolute benefit of 5.4% from chemotherapy.
- The benefit varied with stage (test for trend, P = .04; HR for stage IA, 1.40; 95% CI, 0.95–2.06; HR for stage IB, 0.93; 95% CI, 0.78–1.10; HR for stage II, 0.83; 95% CI, 0.73–0.95; and HR for stage III, 0.83; 95% CI, 0.72–0.94).
- The effect of chemotherapy did not vary significantly (test for interaction, P = .11) with the associated drugs, including vinorelbine (HR, 0.80; 95% CI, 0.70–0.91), etoposide or vinca alkaloid (HR, 0.92; 95% CI, 0.80–1.07), or other drugs (HR, 0.97; 95% CI, 0.84–1.13).
- The greater effect on survival observed with the doublet of cisplatin plus vinorelbine compared with other regimens should be interpreted cautiously as the total dose of cisplatin received was significantly higher in patients treated with vinorelbine.
- The meta-analysis [7] and the individual studies [5,12] support the administration of postoperative cisplatin-based chemotherapy in combination with vinorelbine.
- Superior OS for the trial population and patients with stage II disease was reported for the Lung Adjuvant Cisplatin Evaluation (LACE) pooled analysis (pooled HR, 0.83; 95% CI, 0.73–0.95); the Adjuvant Navelbine International Trialist Association (ANITA) trial (HR, 0.71; 95% CI, 0.49–1.03); and the National Cancer Institute of Canada Clinical Trials Group JBR.10 trial (HR, 0.59; 95% CI, 0.42–0.85).
- Chemotherapy effect was higher in patients with better performance status.
- There was no interaction between chemotherapy effect and any of the following:
- Sex.
- Age.
- Histology.
- Type of surgery.
- Planned radiation therapy.
- Planned total dose of cisplatin.
- In a retrospective analysis of a phase III trial of postoperative cisplatin and vinorelbine, patients older than 65 years were found to benefit from treatment.[13]
- Chemotherapy significantly prolonged OS for patients older than 65 years (HR, 0.61; 95% CI, 0.38–0.98; P = .04).
- There were no significant differences in toxic effects, hospitalization, or treatment-related death by age group, although patients older than 65 years received less treatment.[13]
- Several other randomized controlled trials and meta-analyses have evaluated the use of postoperative chemotherapy in patients with stages I, II, and IIIA NSCLC.[5,6,7,8,9,10,11]
Based on these data, patients with completely resected stage II lung cancer may benefit from postoperative cisplatin-based chemotherapy.[13][Level of evidence A1]
Adjuvant targeted therapy (for patients withEGFRmutations)
Adjuvant targeted therapy with osimertinib for patients with EGFR-mutated NSCLC and resected stage IB to IIIA NSCLC was studied in a phase III clinical trial and showed improved OS.
Evidence (adjuvant targeted therapy with osimertinib for patients with stages IIA and IIB EGFR-mutated NSCLC):
- The ADAURA (NCT02511106) phase III, double-blind, placebo-controlled trial randomly assigned 682 patients with surgically resected stage IB to stage IIIA NSCLC with EGFR-sensitizing mutations (centrally determined, deletion in exon 19 or L858R mutation in exon 21) to receive osimertinib 80 mg by mouth daily or a placebo for 3 years. Standard postoperative adjuvant chemotherapy was allowed but not mandatory; decisions regarding adjuvant chemotherapy were made by the physician and patient before trial enrollment. There were 399 patients who received osimertinib and 342 patients who received placebo.[14][Level of evidence A1]
- In the overall population, the 5-year OS rate was 88% in the osimertinib group and 78% in the placebo group (overall HRdeath, 0.49; 95.03% CI, 0.34–0.70; P < .001).
- Among patients with stage II to IIIA disease, the 5-year OS rate was 85% in the osimertinib group and 73% in the placebo group (overall HRdeath, 0.49; 95.03% CI, 0.33–0.73; P < .001).
- The adverse event profile is consistent with other studies that used osimertinib except for pneumonia related to COVID-19, which was reported later.
The U.S. Food and Drug Administration (FDA) approved osimertinib as adjuvant therapy for patients with stage IB to IIIA NSCLC with EGFR exon 19 deletions or exon 21 L858R mutations.
Adjuvant immunotherapy
Adjuvant immunotherapy for patients with resected stage IB to IIIA NSCLC has been found to significantly increase DFS.[15,16]
Evidence (adjuvant immunotherapy with pembrolizumab for patients with stage IIA and IIB tumors >4 cm):
- The phase III, multicenter, open-label PEARLS/KEYNOTE-091 trial (NCT02504372) randomly assigned 1,177 patients with completely resected stage IB (tumor >4 cm) to stage IIIA NSCLC to receive pembrolizumab (200 mg every 3 weeks) or placebo for up to 18 cycles, or until disease progression or unacceptable toxicity. Patients started study treatment after resection or, if indicated, after adjuvant chemotherapy (maximum of four cycles). The dual primary end points were DFS in the overall study population and DFS in patients with a programmed death-ligand 1 (PD-L1) tumor proportion score (TPS) of 50% or greater, as determined using the PD-L1 IHC 22C3 pharmDx assay. These end points were reported in a prespecified interim analysis after a median follow-up of 35.6 months (interquartile range [IQR], 27.1–45.5).[15][Level of evidence B1]
- In the overall study population, the median DFS was 53.6 months (95% CI, 39.2–not reached [NR]) in the pembrolizumab group and 42.0 months (95% CI, 31.3–NR) in the placebo group (HR, 0.76; 95% CI, 0.63–0.91; P = .0014).
- In the PD-L1 TPS ≥50% population, the median DFS was not reached with either pembrolizumab (95% CI, 44.3–NR) or placebo (95% CI, 35.8–NR) (HR, 0.82; 95% CI, 0.57–1.18; P = .14).
- OS data were immature at the time of the prespecified interim analysis.
- No new safety signals were identified in this study.
The FDA approved pembrolizumab as a single agent for adjuvant treatment following resection and platinum-based chemotherapy for patients with stage IB (T2a ≥4 cm), II, or IIIA NSCLC. Of note, the FDA label specifies that pembrolizumab can be used as adjuvant therapy after platinum-based chemotherapy. However, chemotherapy was not required in the overall study patient population evaluated in KEYNOTE-091.
Evidence (adjuvant immunotherapy with atezolizumab for patients with stages IIA and IIB NSCLC):
- IMpower010 (NCT02486718) was a phase III, multicenter, open-label trial that randomly assigned 1,005 patients with surgically resected stage IB (tumor >4 cm) to stage IIIA NSCLC. Patients received atezolizumab (1,200 mg every 21 days intravenously) or best supportive care for 16 cycles or 1 year after standard adjuvant platinum-based chemotherapy. Patients were enrolled after resection if they were eligible for cisplatin-based chemotherapy and were randomized after completion of chemotherapy if they remained eligible and did not experience disease progression. The primary end point was investigator-assessed DFS.[16]
- The primary end point was tested hierarchically, first in the stage II to IIIA population subgroup whose tumors expressed PD-L1 on at least 1% of tumor cells (using the SP263 antibody), then in all patients in the stage II to IIIA population, and finally in the intention-to-treat (ITT) population (stage IB to IIIA). Of the 882 patients who were randomly assigned and had stage II to IIIA disease, 476 had tumors expressing PD-L1 on at least 1% of tumor cells per SP263.[16][Level of evidence B1]
- After a median follow-up of 32.2 months, atezolizumab treatment improved DFS compared with best supportive care in patients in the stage II to IIIA population whose tumors expressed PD-L1 on at least 1% of tumor cells (HR, 0.66; 95% CI, 0.50–0.88; P = .0039). At 24 months, the DFS rate was 74.6% for the atezolizumab group and 61.0% for the best supportive care group.
- Atezolizumab also improved DFS in all patients in the stage II to IIIA population (HR, 0.79; 95% CI, 0.64–0.96; P = .020). At 24 months, the DFS rate was 70.2% for the atezolizumab group and 61.6% for the best supportive care group.
- In the ITT population, which included patients with stage IB to IIIA disease, HRDFS was 0.81 (95% CI, 0.67–0.99; P = .040). However, the boundary for statistical significance for DFS was not crossed.
- OS data are immature.
- No new safety signals were noted.
The FDA approved atezolizumab for adjuvant treatment of patients with stage II to IIIA NSCLC whose tumors express PD-L1 on at least 1% of tumor cells.
Adjuvant radiation therapy
The value of postoperative (adjuvant) radiation therapy (PORT) has been evaluated.[17]
Evidence (adjuvant radiation therapy):
- A meta-analysis, based on the results of ten randomized controlled trials and 2,232 individuals, reported the following:[17]
- An 18% relative increase in the risk of death for patients who received PORT compared with surgery alone (HR, 1.18; P = .002). This is equivalent to an absolute detriment of 6% at 2 years (95% CI, 2%–9%), reducing OS from 58% to 52%. Exploratory subgroup analyses suggested that this detrimental effect was most pronounced for patients with stage I/II, N0 to N1 disease, whereas for patients with stage III, N2 disease there was no clear evidence of an adverse effect.
- Results for local (HR, 1.13; P = .02), distant (HR, 1.14; P = .02), and overall (HR, 1.10; P = .06) recurrence-free survival similarly showed a detriment of PORT.[17][Level of evidence A1]
Further analysis is needed to determine whether these outcomes can potentially be modified with technical improvements, better definitions of target volumes, and limitation of cardiac volume in the radiation portals.
Neoadjuvant chemotherapy
The role of chemotherapy before surgery was tested in clinical trials. The proposed benefits of preoperative chemotherapy include:
- A reduction in tumor size that may facilitate surgical resection.
- Early eradication of micrometastases.
- Better tolerability.
Preoperative chemotherapy may, however, delay potentially curative surgery.
Evidence (neoadjuvant chemotherapy):
- The Cochrane Collaboration reported a systematic review and meta-analysis of seven randomized controlled trials that included 988 patients and evaluated the addition of preoperative chemotherapy to surgery versus surgery alone. These trials evaluated patients with stages I, II, and IIIA NSCLC.[18]
- Preoperative chemotherapy provided an absolute benefit in survival of 6% across all stages of disease, from 14% to 20% at 5 years (HR, 0.82; 95% CI, 0.69–0.97; P = .022).[18][Level of evidence A1]
- This analysis was unable to address questions such as whether particular types of patients may benefit more or less from preoperative chemotherapy.
- In the largest trial reported to date, 519 patients were randomly assigned to receive either surgery alone or three cycles of platinum-based chemotherapy followed by surgery. Most patients (61%) had clinical stage I disease; 31% had stage II disease; and 7% had stage III disease.[19]
- No survival advantage was seen.[19]
- Postoperative complications were similar between groups, and no impairment of quality of life was observed.
- There was no evidence of a benefit in terms of OS (HR, 1.02; 95% CI, 0.80–1.31; P = .86).
- Updating the systematic review by addition of the present result suggests a 12% relative survival benefit with the addition of neoadjuvant (preoperative) chemotherapy (1,507 patients; HR, 0.88; 95% CI, 0.76–1.01; P = .07), equivalent to an absolute improvement in survival of 5% at 5 years.
Neoadjuvant immunotherapy with chemotherapy
Nivolumab plus platinum-based chemotherapy
The CheckMate 816 trial evaluated the combination of nivolumab (an anti-programmed death 1 antibody) and platinum-based chemotherapy as neoadjuvant therapy in patients with resectable (≥4 cm or node positive) NSCLC. Nivolumab therapy improved event-free survival (EFS) and pathological complete response rates compared with chemotherapy alone.
Evidence (nivolumab plus platinum-based chemotherapy):
- CheckMate 816 (NCT02998528) was a phase III open-label trial that enrolled 358 patients with resectable stage IB to stage IIIA NSCLC. Notably, patients with stage IB disease had tumors measuring at least 4 cm and were classified according to the AJCC 7th edition staging criteria used for this trial; these tumors are now classified as stage IIA according to the AJCC 8th edition staging criteria. Patients were randomly assigned to receive nivolumab (360 mg) in combination with platinum-doublet chemotherapy or platinum-doublet chemotherapy alone every 3 weeks for three cycles before undergoing definitive surgery. The primary end points were EFS (defined as the time from randomization to any progression of disease precluding surgery, progression or recurrence of disease after surgery, progression of disease in the absence of surgery, or death from any cause) and pathological complete response (defined as 0% residual viable tumor cells in the primary tumor and sampled lymph nodes) according to blinded independent central review.[20][Level of evidence B1]
- With a minimum follow-up of 21 months, the median EFS was 31.6 months (95% CI, 30.2–NR) in the nivolumab-plus-chemotherapy group and 20.8 months (95% CI, 14.0–26.7) in the chemotherapy-alone group (HR, 0.63; 97.38% CI, 0.43–0.91; P = .005).
- The estimated percentage of patients surviving without disease progression or disease recurrence at 1 year was 76.1% for patients who received nivolumab plus chemotherapy and 63.4% for patients who received chemotherapy alone. The magnitude of benefit was greater in 1) patients with stage IIIA disease versus patients with stage IB or II disease (HR, 0.54; 95% CI, 0.37–0.80 vs. HR, 0.87; 95% CI, 0.48–1.56), 2) patients with tumor PD-L1 expression ≥1% versus <1% (HR, 0.41; 95% CI, 0.24–0.70 vs. HR, 0.85; 95% CI, 0.54–1.32), and 3) patients with nonsquamous histology versus squamous histology.
- Pathological complete response was observed in 24% (95% CI, 18.0%– 31.0%) of patients who received nivolumab plus chemotherapy and 2.2% (95% CI, 0.6%–5.6%) of patients who received chemotherapy alone (odds ratio [OR], 13.94; 99% CI, 3.49–55.75; P < .001).
- Median OS was not reached in either group (HRdeath, 0.57; 99.67% CI, 0.30–1.07; P = .008).
- Grade 3 or 4 treatment-related adverse events occurred in 33.5% of patients in the nivolumab-plus-chemotherapy group and in 36.9% of patients in the chemotherapy-alone group. Treatment-related adverse events led to treatment discontinuation in 10.2% of patients in the nivolumab-plus-chemotherapy group and in 9.7% of patients in the chemotherapy-alone group.
The FDA approved nivolumab in combination with platinum-doublet chemotherapy for neoadjuvant treatment of patients with resectable (tumors ≥4 cm or node positive) NSCLC.
Perioperative (neoadjuvant and adjuvant) immunotherapy with chemotherapy
Several immune checkpoint inhibitors have been approved by the FDA for select patient populations with potentially resectable NSCLC, either in the neoadjuvant setting (nivolumab) or adjuvant setting (atezolizumab, durvalumab, or pembrolizumab). Ongoing phase III trials are evaluating the role of perioperative immune checkpoint inhibitors. These regimens for patients with potentially resectable stages II to III NSCLC include neoadjuvant immune checkpoint inhibitors with chemotherapy followed by surgery and adjuvant immune checkpoint inhibitors. Compared with neoadjuvant chemotherapy alone, early results from studies of perioperative immune checkpoint inhibitor regimens have shown improvements in several key outcomes including EFS, major pathological response, pathological complete response, and OS.
Perioperative pembrolizumab plus platinum-based chemotherapy
Evidence (neoadjuvant pembrolizumab plus chemotherapy and adjuvant pembrolizumab):
- The phase III double-blind KEYNOTE-671 (NCT03425643) trial randomly assigned 797 patients with untreated stage II, IIIA, or IIIB (≥1 ipsilateral mediastinal node or subcarinal node) NSCLC. Patients received neoadjuvant pembrolizumab (200 mg every 3 weeks) or placebo with cisplatin-based chemotherapy for four cycles, followed by surgery and adjuvant pembrolizumab or placebo for up to 13 cycles, or until disease progression or unacceptable toxicity. The dual primary end points were EFS (defined as the time from randomization to local progression precluding surgery, unresectable tumor, progression or recurrence, or death) and OS. These end points were reported in a prespecified interim analysis after a median follow-up of 25.2 months (range, 7.5–50.6).[21][Level of evidence B1]
- The estimated 24-month EFS rate was 62.4% (95% CI, 56.8%–67.5%) for patients in the pembrolizumab group and 40.6% (95% CI, 34.8%–46.3%) for patients in the placebo group. Median EFS was not reached in the pembrolizumab group (95% CI, 34.1–NR) and was 17.0 months (95% CI, 14.3–22.0) in the placebo group (HRprogression, recurrence, or death, 0.58; 95% CI, 0.46–0.72; P < .001).
- The EFS benefit with pembrolizumab was generally consistent across all subgroups.
- The estimated 24-month OS rate was 80.9% in the pembrolizumab group and 77.6% in the placebo group (P = .02, not meeting the significance criterion).
- Secondary end points were reported as follows:
- Major pathological response occurred in 30.2% of patients in the pembrolizumab group and 11.0% of patients in the placebo group.
- Pathological complete response occurred in 18.1% of patients in the pembrolizumab group and 4.0% of patients in the placebo group.
- Immune-related adverse events of any grade were seen in 25.3% of patients in the pembrolizumab group (grade 3 or greater in 5.8% of patients) and 10.5% of patients in the placebo group (grade 3 or greater in 1.5% of patients).
- The estimated 24-month EFS rate was 62.4% (95% CI, 56.8%–67.5%) for patients in the pembrolizumab group and 40.6% (95% CI, 34.8%–46.3%) for patients in the placebo group. Median EFS was not reached in the pembrolizumab group (95% CI, 34.1–NR) and was 17.0 months (95% CI, 14.3–22.0) in the placebo group (HRprogression, recurrence, or death, 0.58; 95% CI, 0.46–0.72; P < .001).
Perioperative durvalumab plus platinum-based chemotherapy
Evidence (durvalumab plus platinum-based chemotherapy):
- The phase III AEGEAN trial (NCT03800134) investigated perioperative durvalumab plus neoadjuvant chemotherapy compared with neoadjuvant chemotherapy alone in patients with resectable (stage II to IIIB [N2]) NSCLC. Patients received four cycles of treatment every 3 weeks before surgery, followed by adjuvant durvalumab or placebo intravenously every 4 weeks for 12 cycles. The modified intention-to-treat population (740 patients) included all patients who were randomly assigned, excluding patients with documented EGFR or ALK alterations. The first planned interim analysis occurred with 31.9% data maturity and at a median follow-up of 1 year. The primary end points were EFS and pathological complete response.[22][Level of evidence B1]
- At 12 months, the EFS rate was 73.4% for patients who received durvalumab (95% CI, 67.9%–78.1%), and 64.5% for patients who received chemotherapy alone (95% CI, 58.8%–69.6%).
- Pathological complete response was significantly higher with perioperative durvalumab (17.2%), compared with chemotherapy alone (4.3%, P < .001).
- The EFS and pathological complete response benefit were observed regardless of stage and PD-L1 expression.
- The safety profile was consistent with known profiles of durvalumab and chemotherapy.
Perioperative nivolumab plus platinum-based chemotherapy
Evidence (neoadjuvant nivolumab plus chemotherapy and adjuvant nivolumab):
- The phase III randomized CheckMate 77T (NCT04025879) trial, published in abstract form, compared neoadjuvant chemotherapy and nivolumab followed by surgery and adjuvant nivolumab versus neoadjuvant chemotherapy alone in patients with untreated EGFR/ALK wild-type, resectable stage IIA (>4 cm)–IIIB (N2) NSCLC. Patients were stratified by tumor histology, PD-L1 expression, and disease stage. A total of 461 patients were randomly assigned 1:1 to receive either neoadjuvant nivolumab plus platinum-doublet chemotherapy for four cycles, surgery, and adjuvant nivolumab for 12 months or neoadjuvant platinum-doublet chemotherapy alone followed by surgery. The primary end point was EFS, and secondary end points were pathological complete response, major pathological response, OS, and safety.[23]
- In a prespecified interim analysis for EFS, with a median follow-up of 25.4 months, the median EFS was significantly improved for patients who received nivolumab plus chemotherapy/nivolumab (NR; 95% CI, 28.9 months–NR) when compared with patients who received neoadjuvant chemotherapy alone (18.4 months; 95% CI, 13.6–28.1) (HR, 0.58; 97.36% CI, 0.42–0.81; P = .00025).
- Patients who received nivolumab plus chemotherapy/nivolumab also had higher rates of both pathological complete response (25.3% vs. 4.7%; OR, 6.64; 95% CI, 3.40–12.97) and major pathological response (35.4% vs. 12.1%; OR, 4.01; 95% CI, 2.48–6.49) when compared with patients who received chemotherapy alone.
- Definitive surgery rates were similar among groups (78% for nivolumab plus chemotherapy/nivolumab vs. 77% for chemotherapy alone).
- Grade 3 to 4 treatment-related and surgical adverse events were similar among the groups.
Perioperative toripalimab plus platinum-based chemotherapy
Evidence (toripalimab plus platinum-based chemotherapy):
- A phase III randomized trial (Neotorch [NCT04158440]) evaluated the efficacy and safety of toripalimab in combination with neoadjuvant platinum-based chemotherapy followed by maintenance toripalimab versus chemotherapy alone in patients with resectable stage II, IIIA, or IIIB (N2) NSCLC without EGFR or ALK alterations. Patients were stratified by disease stage (II, IIIA, or IIIB), PD-L1 tumor expression status (≥1%, <1%, or not evaluable using the JS311IHC staining assay), planned surgical approach (pneumonectomy or lobectomy), and histological subtype (squamous vs. nonsquamous). A total of 501 patients with stage II to III resectable NSCLC were randomly assigned to receive either (1) toripalimab plus platinum-based chemotherapy for three cycles before surgery and one cycle after surgery followed by single-agent maintenance toripalimab for up to 13 cycles or (2) platinum-based chemotherapy alone for three cycles before surgery and one cycle after surgery. Coprimary end points were EFS (defined as time from randomization to the first documentation of disease progression leading to the inability to operate, postoperative progression, or local or distant recurrence/death from any cause) and major pathological response (≤10% or less viable tumor cells in the tumor bed). Secondary end points included OS, pathological complete response, DFS after surgery, and safety.[24]
- In a prespecified interim analysis of EFS in patients with stage III NSCLC (n = 404) after a median follow-up of 18.3 months (IQR, 12.7–22.5 months), the median EFS was not reached (95% CI, 24.4 months–NR) in the toripalimab group and was 15.1 months (95% CI, 10.6–21.9) in the placebo group (HR, 0.40; 95% CI, 0.28–0.57; P < .001).
- The 1- and 2-year EFS rates were 84.4% and 64.7%, respectively, in the toripalimab group and 57.0% and 38.7%, respectively, in the placebo group. A consistent effect on EFS, favoring toripalimab, was observed in all subgroups.
- After surgical resection, a major pathological response occurred in 98 patients (48.5%) in the toripalimab group and 17 patients (8.4%) in the placebo group (between group difference, 40.2%; 95% CI, 32.2%–48.1%; P < .001).
The FDA has not approved this drug for patients with lung cancer.
Radiation therapy
Patients with potentially operable tumors with medical contraindications to surgery or those with inoperable stage II disease and with sufficient pulmonary reserve are candidates for radiation therapy with curative intent.[25] Primary radiation therapy often consists of approximately 60 Gy delivered with megavoltage equipment to the midplane of the volume of the known tumor using conventional fractionation. A boost to the cone down field of the primary tumor is frequently used to enhance local control. Careful treatment planning with precise definition of target volume and avoidance of critical normal structures, to the extent possible, is needed for optimal results; this requires the use of a simulator.
Among patients with excellent performance status, a 3-year survival rate of 20% may be expected if a course of radiation therapy with curative intent can be completed.
Evidence (radiation therapy):
- In the largest retrospective series reported to date, 152 patients with medically inoperable NSCLC were treated with definitive radiation therapy. The study reported the following:[26]
- A 5-year OS rate of 10%.
- Forty-four patients with T1 tumors achieved an actuarial DFS rate of 60%.
- This retrospective study also suggested that improved DFS was obtained with radiation therapy doses greater than 60 Gy.[26]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Manser R, Wright G, Hart D, et al.: Surgery for early stage non-small cell lung cancer. Cochrane Database Syst Rev (1): CD004699, 2005.
- Allen MS, Darling GE, Pechet TT, et al.: Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 81 (3): 1013-9; discussion 1019-20, 2006.
- Darling GE, Allen MS, Decker PA, et al.: Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 141 (3): 662-70, 2011.
- Martini N, Bains MS, Burt ME, et al.: Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 109 (1): 120-9, 1995.
- Winton T, Livingston R, Johnson D, et al.: Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352 (25): 2589-97, 2005.
- Arriagada R, Bergman B, Dunant A, et al.: Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350 (4): 351-60, 2004.
- Pignon JP, Tribodet H, Scagliotti GV, et al.: Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 26 (21): 3552-9, 2008.
- Scagliotti GV, Fossati R, Torri V, et al.: Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst 95 (19): 1453-61, 2003.
- Hotta K, Matsuo K, Ueoka H, et al.: Role of adjuvant chemotherapy in patients with resected non-small-cell lung cancer: reappraisal with a meta-analysis of randomized controlled trials. J Clin Oncol 22 (19): 3860-7, 2004.
- Edell ES, Cortese DA: Photodynamic therapy in the management of early superficial squamous cell carcinoma as an alternative to surgical resection. Chest 102 (5): 1319-22, 1992.
- Corti L, Toniolo L, Boso C, et al.: Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med 39 (5): 394-402, 2007.
- Douillard JY, Rosell R, De Lena M, et al.: Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 7 (9): 719-27, 2006.
- Pepe C, Hasan B, Winton TL, et al.: Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol 25 (12): 1553-61, 2007.
- Tsuboi M, Herbst RS, John T, et al.: Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N Engl J Med 389 (2): 137-147, 2023.
- O'Brien M, Paz-Ares L, Marreaud S, et al.: Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 23 (10): 1274-1286, 2022.
- Felip E, Altorki N, Zhou C, et al.: Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398 (10308): 1344-1357, 2021.
- PORT Meta-analysis Trialists Group: Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev (2): CD002142, 2005.
- Burdett SS, Stewart LA, Rydzewska L: Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. Cochrane Database Syst Rev (3): CD006157, 2007.
- Gilligan D, Nicolson M, Smith I, et al.: Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 369 (9577): 1929-37, 2007.
- Forde PM, Spicer J, Lu S, et al.: Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 386 (21): 1973-1985, 2022.
- Wakelee H, Liberman M, Kato T, et al.: Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 389 (6): 491-503, 2023.
- Heymach JV, Harpole D, Mitsudomi T, et al.: Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J Med 389 (18): 1672-1684, 2023.
- Cascone T, Awad MM, Spicer JD, et al.: CheckMate 77T: Phase III study comparing neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) vs neoadjuvant placebo plus chemo followed by surgery and adjuvant NIVO or placebo for previously untreated, resectable stage II–IIIb NSCLC. [Abstract] Ann Oncol 34 (Suppl 2): A-LBA1, S1295, 2023.
- Lu S, Zhang W, Wu L, et al.: Perioperative Toripalimab Plus Chemotherapy for Patients With Resectable Non-Small Cell Lung Cancer: The Neotorch Randomized Clinical Trial. JAMA 331 (3): 201-211, 2024.
- Komaki R, Cox JD, Hartz AJ, et al.: Characteristics of long-term survivors after treatment for inoperable carcinoma of the lung. Am J Clin Oncol 8 (5): 362-70, 1985.
- Dosoretz DE, Katin MJ, Blitzer PH, et al.: Radiation therapy in the management of medically inoperable carcinoma of the lung: results and implications for future treatment strategies. Int J Radiat Oncol Biol Phys 24 (1): 3-9, 1992.
Treatment of Stage IIIA NSCLC
Patients with stage IIIA non-small cell lung cancer (NSCLC) are a heterogenous group. Patients may have metastases to ipsilateral mediastinal nodes, potentially resectable T3 tumors invading the chest wall, or mediastinal involvement with metastases to peribronchial or hilar lymph nodes (N1). Presentations of disease range from resectable tumors with microscopic metastases to lymph nodes to unresectable, bulky disease involving multiple nodal stations.
Patients with clinical stage IIIA N2 disease have a 5-year overall survival (OS) rate of 10% to 15%; however, patients with bulky mediastinal involvement (i.e., visible on chest radiography) have a 5-year survival rate of 2% to 5%. Depending on clinical circumstances, the principal forms of treatment that are considered for patients with stage IIIA NSCLC are radiation therapy, chemotherapy, surgery, and combinations of these modalities.
Treatment options vary according to the location of the tumor and whether it is resectable.
Treatment Options for Resected/Resectable Stage IIIA NSCLC
Treatment options for resected/resectable disease include:
- Surgery with neoadjuvant or adjuvant therapy.
- Neoadjuvant therapy.
- Perioperative (neoadjuvant and adjuvant) immunotherapy with chemotherapy.
- Adjuvant therapy.
- Adjuvant chemotherapy.
- Adjuvant targeted therapy (for patients with EGFR mutations).
- Adjuvant immunotherapy.
- Adjuvant chemoradiation therapy.
- Adjuvant radiation therapy.
Despite careful preoperative staging, some patients will be found to have metastases to mediastinal N2 lymph nodes at thoracotomy.
The preponderance of evidence indicates that postoperative cisplatin combination chemotherapy provides a significant survival advantage to patients with resected NSCLC with occult N2 disease discovered at surgery. The optimal sequence of surgery and chemotherapy and the benefits and risks of postoperative radiation therapy in patients with resectable NSCLC are yet to be determined.
Surgery
If complete resection of tumor and lymph nodes is possible, such patients may benefit from surgery followed by postoperative chemotherapy. Current evidence suggests that lung cancer resection combined with complete ipsilateral mediastinal lymph node dissection (CMLND) is not associated with improvement in survival compared with lung cancer resection combined with systematic sampling of mediastinal lymph nodes in patients with stage I, II, or IIIA NSCLC.[1][Level of evidence A1]
The addition of surgery to chemoradiation therapy for patients with stage IIIA NSCLC did not result in improved OS in a phase III trial but did improve progression-free survival (PFS) and local control.[2][Level of evidence B1]
Evidence (surgery):
- The Cochrane Collaboration reviewed 11 randomized trials with a total of 1,910 patients who underwent surgical interventions for early-stage (I–IIIA) lung cancer.[3] A pooled analysis of three trials reported the following:
- Four-year survival was superior in patients with resectable stage I, II, or IIIA NSCLC who underwent resection and CMLND, compared with those who underwent resection and lymph node sampling; the hazard ratio (HR) was estimated to be 0.78 (95% confidence interval [CI], 0.65–0.93; P = .005).[3][Level of evidence A1]
- CMLND versus lymph node sampling was evaluated in a large randomized phase III trial (ACOSOG-Z0030). Preliminary analyses of operative morbidity and mortality showed comparable rates from the procedures.[4]
- There was no difference in OS, disease-free survival (DFS), local recurrence, and regional recurrence.[1][Level of evidence A1]
Conclusions about the efficacy of surgery for patients with local and locoregional NSCLC are limited by the small number of participants studied to date and by the potential methodological weaknesses of the trials.
Neoadjuvant therapy
Neoadjuvant chemotherapy
The role of chemotherapy before surgery in patients with stage IIIA NSCLC has been extensively tested in clinical trials. The proposed benefits of preoperative (neoadjuvant) chemotherapy include:
- A reduction in tumor size that may facilitate surgical resection.
- Early eradication of micrometastases.
- Better tolerability.
Evidence (neoadjuvant chemotherapy):
- The Cochrane Collaboration provided a systematic review and meta-analysis of seven randomized controlled trials that included 988 patients and evaluated the addition of preoperative chemotherapy to surgery versus surgery alone.[5] These trials evaluated patients with stages I, II, and IIIA NSCLC.
- Preoperative chemotherapy provided an absolute benefit in survival of 6% across all stages of disease, from 14% to 20% at 5 years (HR, 0.82; 95% CI, 0.69–0.97; P = .022).[5][Level of evidence A1]
- This analysis was unable to address questions such as whether particular types of patients may benefit more or less from preoperative chemotherapy.[6]
- In the largest trial reported to date, 519 patients were randomly assigned to receive either surgery alone or three cycles of platinum-based chemotherapy followed by surgery.[7] Most patients (61%) had clinical stage I disease, 31% had stage II disease, and 7% had stage III disease.
- Postoperative complications were similar between groups, and no impairment of quality of life was observed.
- There was no evidence of a benefit in terms of OS (HR, 1.02; 95% CI, 0.80–1.31; P = .86).
- Updating the systematic review by addition of the present result suggests a 12% relative survival benefit with the addition of preoperative chemotherapy (1,507 patients; HR, 0.88; 95% CI, 0.76–1.01; P = .07), equivalent to an absolute improvement in survival of 5% at 5 years.[7]
Neoadjuvant chemoradiation therapy
Administering concurrent neoadjuvant chemotherapy and radiation therapy before surgery may intensify treatment and increase the likelihood of downstaging the tumor burden. Commonly used regimens that have been tested in the phase II setting include cisplatin/etoposide (EP5050) and weekly carboplatin/paclitaxel.[8,9] In a randomized trial of neoadjuvant chemoradiation therapy and surgery versus concurrent chemoradiation therapy alone, there was no difference in OS, but surgery improved PFS and local control.[2][Level of evidence B1]
Evidence (neoadjuvant chemoradiation therapy):
- The Intergroup-0139 trial (NCT00002550) compared chemoradiation therapy alone with neoadjuvant chemoradiation followed by surgery in 396 patients with stage IIIA (N2) NSCLC.[2]
- Surgery did not improve OS (5-year OS rate, 27% vs. 20%; HR, 0.87; 0.70–1.10; P = .24).
- Surgery improved PFS (5-year PFS rate, 22% vs. 11%; HR, 0.77; 0.62–0.96; P = .017) and decreased the risk of local recurrence (10% vs. 22%; P = .002).
- There was increased treatment mortality with neoadjuvant chemoradiation with surgery (8% vs. 2%), particularly in the subset of patients who underwent pneumonectomy.
A direct comparison of neoadjuvant chemotherapy versus neoadjuvant chemoradiation therapy using modern treatment regimens has not been performed to date; the optimal neoadjuvant approach remains unclear.
Neoadjuvant immunotherapy with chemotherapy
Nivolumab plus platinum-based chemotherapy
The CheckMate 816 trial evaluated the combination of nivolumab (an anti-programmed death 1 antibody) and platinum-based chemotherapy as neoadjuvant therapy in patients with resectable (≥4 cm or node positive) NSCLC. Nivolumab therapy improved event-free survival (EFS) and pathological complete response rates compared with chemotherapy alone.
Evidence (nivolumab plus platinum-based chemotherapy):
- CheckMate 816 (NCT02998528) was a phase III open-label trial that enrolled 358 patients with resectable stage IB to stage IIIA NSCLC. Notably, patients with stage IB disease had tumors measuring at least 4 cm and were classified according to the AJCC 7th edition staging criteria used for this trial; these tumors are now classified as stage IIA according to the AJCC 8th edition staging criteria. Patients were randomly assigned to receive nivolumab (360 mg) in combination with platinum-doublet chemotherapy or platinum-doublet chemotherapy alone every 3 weeks for three cycles before undergoing definitive surgery. The primary end points were EFS (defined as the time from randomization to any progression of disease precluding surgery, progression or recurrence of disease after surgery, progression of disease in the absence of surgery, or death from any cause) and pathological complete response (defined as 0% residual viable tumor cells in the primary tumor and sampled lymph nodes) according to blinded independent central review.[10][Level of evidence B1]
- With a minimum follow-up of 21 months, the median EFS was 31.6 months (95% CI, 30.2–not reached [NR]) in the nivolumab-plus-chemotherapy group and 20.8 months (95% CI, 14.0–26.7) in the chemotherapy-alone group (HR, 0.63; 97.38% CI, 0.43–0.91; P = .005).
- The estimated percentage of patients surviving without disease progression or disease recurrence at 1 year was 76.1% for patients who received nivolumab plus chemotherapy and 63.4% for patients who received chemotherapy alone. The magnitude of benefit was greater in 1) patients with stage IIIA disease versus patients with stage IB or II disease (HR, 0.54; 95% CI, 0.37–0.80 vs. HR, 0.87; 95% CI, 0.48–1.56), 2) patients with tumor programmed death-ligand 1 (PD-L1) expression ≥1% versus <1% (HR, 0.41; 95% CI, 0.24–0.70 vs. HR, 0.85; 95% CI, 0.54–1.32), and 3) patients with nonsquamous histology versus squamous histology.
- Pathological complete response was observed in 24% (95% CI, 18.0%– 31.0%) of patients who received nivolumab plus chemotherapy and 2.2% (95% CI, 0.6%–5.6%) of patients who received chemotherapy alone (odds ratio, 13.94; 99% CI, 3.49–55.75; P < .001).
- Median OS was not reached in either group (HRdeath, 0.57; 99.67% CI, 0.30–1.07; P = .008).
- Grade 3 or 4 treatment-related adverse events occurred in 33.5% of patients in the nivolumab-plus-chemotherapy group and in 36.9% of patients in the chemotherapy-alone group. Treatment-related adverse events led to treatment discontinuation in 10.2% of patients in the nivolumab-plus-chemotherapy group and in 9.7% of patients in the chemotherapy-alone group.
- NADIM (NCT03081689) was an open-label, multicenter, single-arm trial for patients with stage IIIA NSCLC with surgically resectable disease. Patients received neoadjuvant paclitaxel and carboplatin plus nivolumab (360 mg) every 21 days for three cycles, followed by adjuvant nivolumab monotherapy for 1 year (240 mg once every 2 weeks for 4 months, followed by 480 mg once every 4 weeks for 8 months). The intention-to-treat (ITT) population included all patients who received neoadjuvant treatment. The per-protocol population included all patients who underwent tumor resection and received at least one cycle of adjuvant therapy. The median follow-up time was 38.0 months (95% CI, 36.7–40.7), with 94% maturity at 36 months and 90% maturity at 42 months.[11]
- The OS rate at 36 months was 81.9% (95% CI, 66.8%–90.6%) in the ITT population and 91% (95% CI, 74.2%–97.0%) in the per-protocol population.[11][Level of evidence A1]
- In the ITT population, the PFS rate was 69.6% (95% CI, 54.1%–80.7%) at 36 and 42 months. In the per-protocol population, the PFS rate was 81.1% (95% CI, 64.4%–90.5%) at 36 and 42 months.
- Major pathological response was defined as the presence of 10% or fewer tumor cells in the primary tumor. A total of 82.9% of patients had a major pathological response, including 63.4% of patients who had a complete pathological response. A total of 17.1% patients had an incomplete response.
- Tumor mutational burden and PD-L1 status were not predictive of survival.
Circulating tumor DNA (ctDNA) from plasma samples obtained before and after neoadjuvant treatment (but before surgery) was analyzed with the hybridization capture–based TruSight Oncology 500 ctDNA next-generation sequencing assay on a NovaSeq sequencer (Illumina).
- Low pretreatment levels of ctDNA were significantly associated with improved PFS (HR, 0.20; 95% CI, 0.06–0.63) and OS (HR, 0.07; 95% CI, 0.01–0.39).
- Undetectable ctDNA levels after neoadjuvant treatment were significantly associated with PFS (HR, 0.26; 95% CI, 0.07–0.93) and OS (HR, 0.04; 95% CI, 0.00–0.55).
The U.S. Food and Drug Administration (FDA) approved nivolumab in combination with platinum-doublet chemotherapy for neoadjuvant treatment of patients with resectable (tumors ≥4 cm or node positive) NSCLC.
Perioperative (neoadjuvant and adjuvant) immunotherapy with chemotherapy
Several immune checkpoint inhibitors have been approved by the FDA for select patient populations with potentially resectable NSCLC, either in the neoadjuvant setting (nivolumab) or adjuvant setting (atezolizumab, durvalumab, or pembrolizumab). Ongoing phase III trials are evaluating the role of perioperative immune checkpoint inhibitors. These regimens for patients with potentially resectable stages II to III NSCLC include neoadjuvant immune checkpoint inhibitors with chemotherapy followed by surgery and adjuvant immune checkpoint inhibitors. Compared with neoadjuvant chemotherapy alone, early results from studies of perioperative immune checkpoint inhibitor regimens have shown improvements in several key outcomes including EFS, major pathological response, pathological complete response, and OS.
Perioperative pembrolizumab plus platinum-based chemotherapy
Evidence (neoadjuvant pembrolizumab plus chemotherapy and adjuvant pembrolizumab):
- The phase III double-blind KEYNOTE-671 (NCT03425643) trial randomly assigned 797 patients with untreated stage II, IIIA, or IIIB (≥1 ipsilateral mediastinal node or subcarinal node) NSCLC. Patients received neoadjuvant pembrolizumab (200 mg every 3 weeks) or placebo with cisplatin-based chemotherapy for four cycles, followed by surgery and adjuvant pembrolizumab or placebo for up to 13 cycles, or until disease progression or unacceptable toxicity. The dual primary end points were EFS (defined as the time from randomization to local progression precluding surgery, unresectable tumor, progression or recurrence, or death) and OS. These end points were reported in a prespecified interim analysis after a median follow-up of 25.2 months (range, 7.5–50.6).[12][Level of evidence B1]
- The estimated 24-month EFS rate was 62.4% (95% CI, 56.8%–67.5%) for patients in the pembrolizumab group and 40.6% (95% CI, 34.8%–46.3%) for patients in the placebo group. Median EFS was not reached in the pembrolizumab group (95% CI, 34.1–NR) and was 17.0 months (95% CI, 14.3–22.0) in the placebo group (HRprogression, recurrence, or death, 0.58; 95% CI, 0.46–0.72; P < .001).
- The EFS benefit with pembrolizumab was generally consistent across all subgroups.
- The estimated 24-month OS rate was 80.9% in the pembrolizumab group and 77.6% in the placebo group (P = .02, not meeting the significance criterion).
- Secondary end points were reported as follows:
- Major pathological response occurred in 30.2% of patients in the pembrolizumab group and 11.0% of patients in the placebo group.
- Pathological complete response occurred in 18.1% of patients in the pembrolizumab group and 4.0% of patients in the placebo group.
- Immune-related adverse events of any grade were seen in 25.3% of patients in the pembrolizumab group (grade 3 or greater in 5.8% of patients) and 10.5% of patients in the placebo group (grade 3 or greater in 1.5% of patients).
- The estimated 24-month EFS rate was 62.4% (95% CI, 56.8%–67.5%) for patients in the pembrolizumab group and 40.6% (95% CI, 34.8%–46.3%) for patients in the placebo group. Median EFS was not reached in the pembrolizumab group (95% CI, 34.1–NR) and was 17.0 months (95% CI, 14.3–22.0) in the placebo group (HRprogression, recurrence, or death, 0.58; 95% CI, 0.46–0.72; P < .001).
Perioperative durvalumab plus platinum-based chemotherapy
Evidence (durvalumab plus platinum-based chemotherapy):
- The phase III AEGEAN trial (NCT03800134) investigated perioperative durvalumab plus neoadjuvant chemotherapy compared with neoadjuvant chemotherapy alone in patients with resectable (stage II to IIIB [N2]) NSCLC. Patients received four cycles of treatment every 3 weeks before surgery, followed by adjuvant durvalumab or placebo intravenously every 4 weeks for 12 cycles. The modified intention-to-treat population (740 patients) included all patients who were randomly assigned, excluding patients with documented EGFR or ALK alterations. The first planned interim analysis occurred with 31.9% data maturity and at a median follow-up of 1 year. The primary end points were EFS and pathological complete response.[13][Level of evidence B1]
- At 12 months, the EFS rate was 73.4% for patients who received durvalumab (95% CI, 67.9%–78.1%), and 64.5% for patients who received chemotherapy alone (95% CI, 58.8%–69.6%).
- Pathological complete response was significantly higher with perioperative durvalumab (17.2%), compared with chemotherapy alone (4.3%, P < .001).
- The EFS and pathological complete response benefit were observed regardless of stage and PD-L1 expression.
- The safety profile was consistent with known profiles of durvalumab and chemotherapy.
Perioperative nivolumab plus platinum-based chemotherapy
Evidence (neoadjuvant nivolumab plus chemotherapy and adjuvant nivolumab):
- The phase III randomized CheckMate 77T (NCT04025879) trial, published in abstract form, compared neoadjuvant chemotherapy and nivolumab followed by surgery and adjuvant nivolumab versus neoadjuvant chemotherapy alone in patients with untreated EGFR/ALK wild-type, resectable stage IIA (>4 cm)–IIIB (N2) NSCLC. Patients were stratified by tumor histology, PD-L1 expression, and disease stage. A total of 461 patients were randomly assigned 1:1 to receive either neoadjuvant nivolumab plus platinum-doublet chemotherapy for four cycles, surgery, and adjuvant nivolumab for 12 months or neoadjuvant platinum-doublet chemotherapy alone followed by surgery. The primary end point was EFS, and secondary end points were pathological complete response, major pathological response, OS, and safety.[14]
- In a prespecified interim analysis for EFS, with a median follow-up of 25.4 months, the median EFS was significantly improved for patients who received nivolumab plus chemotherapy/nivolumab (NR; 95% CI, 28.9 months–NR) when compared with patients who received neoadjuvant chemotherapy alone (18.4 months; 95% CI, 13.6–28.1) (HR, 0.58; 97.36% CI, 0.42–0.81; P = .00025).
- Patients who received nivolumab plus chemotherapy/nivolumab also had higher rates of both pathological complete response (25.3% vs. 4.7%; OR, 6.64; 95% CI, 3.40–12.97) and major pathological response (35.4% vs. 12.1%; OR, 4.01; 95% CI, 2.48–6.49) when compared with patients who received chemotherapy alone.
- Definitive surgery rates were similar among groups (78% for nivolumab plus chemotherapy/nivolumab vs. 77% for chemotherapy alone).
- Grade 3 to 4 treatment-related and surgical adverse events were similar among the groups.
Perioperative toripalimab plus platinum-based chemotherapy
Evidence (toripalimab plus platinum-based chemotherapy):
- A phase III randomized trial (Neotorch [NCT04158440]) evaluated the efficacy and safety of toripalimab in combination with neoadjuvant platinum-based chemotherapy followed by maintenance toripalimab versus chemotherapy alone in patients with resectable stage II, IIIA, or IIIB (N2) NSCLC without EGFR or ALK alterations. Patients were stratified by disease stage (II, IIIA, or IIIB), PD-L1 tumor expression status (≥1%, <1%, or not evaluable using the JS311IHC staining assay), planned surgical approach (pneumonectomy or lobectomy), and histological subtype (squamous vs. nonsquamous). A total of 501 patients with stage II to III resectable NSCLC were randomly assigned to receive either (1) toripalimab plus platinum-based chemotherapy for three cycles before surgery and one cycle after surgery followed by single-agent maintenance toripalimab for up to 13 cycles or (2) platinum-based chemotherapy alone for three cycles before surgery and one cycle after surgery. Coprimary end points were EFS (defined as time from randomization to the first documentation of disease progression leading to the inability to operate, postoperative progression, or local or distant recurrence/death from any cause) and major pathological response (≤10% or less viable tumor cells in the tumor bed). Secondary end points included OS, pathological complete response, DFS after surgery, and safety.[15]
- In a prespecified interim analysis of EFS in patients with stage III NSCLC (n = 404) after a median follow-up of 18.3 months (IQR, 12.7–22.5 months), the median EFS was not reached (95% CI, 24.4 months–NR) in the toripalimab group and was 15.1 months (95% CI, 10.6–21.9) in the placebo group (HR, 0.40; 95% CI, 0.28–0.57; P < .001).
- The 1- and 2-year EFS rates were 84.4% and 64.7%, respectively, in the toripalimab group and 57.0% and 38.7%, respectively, in the placebo group. A consistent effect on EFS, favoring toripalimab, was observed in all subgroups.
- After surgical resection, a major pathological response occurred in 98 patients (48.5%) in the toripalimab group and 17 patients (8.4%) in the placebo group (between group difference, 40.2%; 95% CI, 32.2%–48.1%; P < .001).
The FDA has not approved this drug for patients with lung cancer.
Adjuvant therapy
Adjuvant chemotherapy
Patients with completely resected stage IIIA NSCLC may benefit from postoperative cisplatin-based chemotherapy.[16][Level of evidence A1]
Evidence (adjuvant chemotherapy):
Evidence from randomized controlled clinical trials indicates that when stage IIIA NSCLC is encountered unexpectedly at surgery, chemotherapy given after complete resection improves survival.
Several randomized, controlled trials and meta-analyses have evaluated the use of postoperative chemotherapy in patients with stages I, II, and IIIA NSCLC.[16,17,18,19,20,21,22]
- Data on individual patient outcomes from the five largest trials (4,584 patients) that were conducted after 1995 of cisplatin-based chemotherapy in patients with completely resected NSCLC were collected and pooled into a meta-analysis.[16]
- With a median follow-up of 5.2 years, the overall HRdeath was 0.89 (95% CI, 0.82–0.96; P = .005), corresponding to a 5-year absolute benefit of 5.4% from chemotherapy.
- The effect of chemotherapy did not vary significantly (test for interaction, P = .11) with the associated drugs, including vinorelbine (HR, 0.80; 95% CI, 0.70–0.91), etoposide or vinca alkaloid (HR, 0.92; 95% CI, 0.80–1.07), or other drugs (HR, 0.97; 95% CI, 0.84–1.13).
- The benefit varied with stage (HR for stage IIIA, 0.83; 95% CI, 0.72–0.94).
- The greater effect on survival observed with the doublet of cisplatin plus vinorelbine compared with other regimens should be interpreted with caution as the total dose of cisplatin received was significantly higher in patients treated with vinorelbine.
- Two trials (FRE-IALT and the Adjuvant Navelbine International Trialist Association [ANITA] trial) reported significant OS benefits associated with postoperative chemotherapy in stage IIIA disease.[6,18]
- For the subgroup of stage IIIA patients in the ANITA trial (n = 325), the HR was 0.69 (95% CI, 0.53–0.90), and the result for the FRE-IALT trial (n = 728) was HR, 0.79 (95% CI, 0.66–0.95).
- The chemotherapy effect was higher in patients with a better performance status.
- There was no interaction between the chemotherapy effect and any of the following:
- Sex.
- Age.
- Histology.
- Type of surgery.
- Planned radiation therapy.
- Planned total dose of cisplatin.
- In a retrospective analysis of a phase III trial of postoperative cisplatin and vinorelbine, patients older than 65 years were found to benefit from treatment.[23]
- Chemotherapy significantly prolonged OS for patients older than 65 years (HR, 0.61; 95% CI, 0.38–0.98; P = .04).
- There were no significant differences in toxic effects, hospitalization, or treatment-related death by age group, although patients older than 65 years received less treatment.
Adjuvant targeted therapy (for patients withEGFRmutations)
Adjuvant targeted therapy with osimertinib for patients with EGFR-mutated NSCLC and resected stage IB to IIIA NSCLC was studied in a phase III clinical trial and showed improved OS.
Evidence (adjuvant targeted therapy with osimertinib for patients with stage IIIA EGFR-mutated NSCLC):
- The ADAURA (NCT02511106) phase III, double-blind, placebo-controlled trial randomly assigned 682 patients with surgically resected stage IB to stage IIIA NSCLC with EGFR-sensitizing mutations (centrally determined, deletion in exon 19 or L858R mutation in exon 21) to osimertinib 80 mg by mouth daily or a placebo for 3 years. Standard postoperative adjuvant chemotherapy was allowed but not mandatory; decisions regarding adjuvant chemotherapy were made by the physician and patient before trial enrollment. There were 399 patients who received osimertinib and 342 patients who received placebo.[24][Level of evidence A1]
- In the overall population, the 5-year OS rate was 88% in the osimertinib group and 78% in the placebo group (overall HRdeath, 0.49; 95.03% CI, 0.34–0.70; P < .001).
- Among patients with stage II to IIIA disease, the 5-year OS rate was 85% in the osimertinib group and 73% in the placebo group (overall HRdeath, 0.49; 95.03% CI, 0.33–0.73; P < .001).
- The adverse event profile is consistent with other studies that used osimertinib except for pneumonia related to COVID-19, which was reported later.
The FDA approved osimertinib as adjuvant therapy for patients with stage IB to IIIA NSCLC with EGFR exon 19 deletions or exon 21 L858R mutations.
Adjuvant immunotherapy
Adjuvant immunotherapy for patients with resected stage IB to IIIA NSCLC has been found to significantly increase DFS.[25,26]
Evidence (adjuvant immunotherapy with pembrolizumab for patients with stage IIIA NSCLC):
- The phase III, multicenter, open-label PEARLS/KEYNOTE-091 trial (NCT02504372) randomly assigned 1,177 patients with completely resected stage IB (tumor >4 cm) to stage IIIA NSCLC to receive pembrolizumab (200 mg every 3 weeks) or placebo for up to 18 cycles, or until disease progression, or unacceptable toxicity. Patients started study treatment after resection or, if indicated, after adjuvant chemotherapy (maximum of four cycles). The dual primary end points were DFS in the overall study population and DFS in patients with a PD-L1 tumor proportion score (TPS) of 50% or greater, as determined using the PD-L1 IHC 22C3 pharmDx assay. These end points were reported in a prespecified interim analysis after a median follow-up of 35.6 months (interquartile range, 27.1–45.5).[25][Level of evidence B1]
- In the overall study population, the median DFS was 53.6 months (95% CI, 39.2 to NR) in the pembrolizumab group and 42.0 months (95% CI, 31.3–NR) in the placebo group (HR, 0.76; 95% CI, 0.63–0.91, P = .0014).
- In the PD-L1 TPS ≥50% population, the median DFS was not reached with either pembrolizumab (95% CI, 44.3–NR) or placebo (95% CI, 35.8–NR) (HR, 0.82; 95% CI, 0.57–1.18; P = .14).
- OS data were immature at the time of prespecified interim analysis.
- No new safety signals were identified in this study.
The FDA approved pembrolizumab as a single agent for adjuvant treatment following resection and platinum-based chemotherapy for patients with stage IB (T2a ≥4 cm), II, or IIIA NSCLC. Of note, the FDA label specifies that pembrolizumab can be used as adjuvant therapy after platinum-based chemotherapy. However, chemotherapy was not required in the overall study patient population evaluated in KEYNOTE-091.
Evidence (adjuvant immunotherapy with atezolizumab for patients with resected stage IIIA NSCLC):
- IMpower010 (NCT02486718) was a phase III, multicenter, open-label trial that randomly assigned 1,005 patients with surgically resected stage IB (tumor >4 cm) to stage IIIA NSCLC. Patients received atezolizumab (1,200 mg every 21 days intravenously) or best supportive care for 16 cycles or 1 year after standard adjuvant platinum-based chemotherapy. Patients were enrolled after resection if they were eligible for cisplatin-based chemotherapy and were randomized after completion of chemotherapy if they remained eligible and did not experience disease progression. The primary end point was investigator-assessed DFS.[26]
- The primary end point was tested hierarchically, first in the stage II to IIIA population subgroup whose tumors expressed PD-L1 on at least 1% of tumor cells (using the SP263 antibody), then in all patients in the stage II to IIIA population, and finally in the ITT population (stage IB to IIIA). Of the 882 patients who were randomly assigned and had stage II to IIIA disease, 476 had tumors expressing PD-L1 on at least 1% of tumor cells per SP263.[26][Level of evidence B1]
- After a median follow-up of 32.2 months, atezolizumab treatment improved DFS compared with best supportive care in patients in the stage II to IIIA population whose tumors expressed PD-L1 on at least 1% of tumor cells (HR, 0.66; 95% CI, 0.50–0.88; P = .0039). At 24 months, the DFS rate was 74.6% for the atezolizumab group and 61.0% for the best supportive care group.
- Atezolizumab also improved DFS in all patients in the stage II to IIIA population (HR, 0.79; 95% CI, 0.64–0.96; P = .020). At 24 months, the DFS rate was 70.2% for the atezolizumab group and 61.6% for the best supportive care group.
- In the ITT population, which included patients with stage IB to IIIA disease, HRDFS was 0.81 (95% CI, 0.67–0.99; P = .040). However, the boundary for statistical significance for DFS was not crossed.
- OS data are immature.
- No new safety signals were noted.
The FDA approved atezolizumab for adjuvant treatment of patients with stage II to IIIA NSCLC whose tumors express PD-L1 on at least 1% of tumor cells.
Adjuvant chemoradiation therapy
Combination chemotherapy and radiation therapy administered before or following surgery should be viewed as investigational and requiring evaluation in future clinical trials.
Evidence (adjuvant chemoradiation therapy):
- Five randomized trials have assessed the value of postoperative combination chemoradiation therapy versus radiation therapy following surgical resection.[5,7,27,28,29][Level of evidence A1]
- Only one trial reported improved DFS, and no trial reported improved OS.
- Three trials have evaluated platinum-based combination chemotherapy followed by surgery versus platinum-based chemotherapy followed by radiation therapy (60–69.6 Gy) alone to determine whether surgery or radiation therapy was most efficacious.[29,30,31] Although the studies were small, enrolling 73 (Radiation Therapy Oncology Group [RTOG]) (RTOG 89-01), 107 (The University of Texas M.D. Anderson Cancer Center), and 333 (European Organisation for Research and Treatment of Cancer [EORTC-08941; NCT00002623]) patients with stage IIIA (N2) disease, no trial reported a difference in local control or survival.[29,30,31][Level of evidence A1]
- In the largest series (EORTC-08941), 579 patients with histologically- or cytologically-proven stage IIIA (N2) NSCLC were given three cycles of platinum-based induction chemotherapy.[31] The 333 responding patients were subsequently randomly assigned to surgical resection or radiation therapy. Of the 154 patients (92%) who underwent surgery, 50% had a radical resection, 42% had a pathological downstaging, and 5% had a pathological complete response; 4% died after surgery. Postoperative (adjuvant) radiation therapy (PORT) was administered to 62 patients (40%) in the surgery arm. Among the 154 patients (93%) who received radiation therapy, overall compliance to the radiation therapy prescription was 55%, and grade 3 to 4 acute and late esophageal and pulmonary toxic effects occurred in 4% and 7% of patients; one patient died of radiation pneumonitis.
- Median OS was 16.4 months for patients assigned to resection versus 17.5 months for patients assigned to radiation therapy; the 5-year OS rate was 15.7% for patients assigned to resection versus 14% for patients assigned to radiation therapy (HR, 1.06; 95% CI, 0.84–1.35).[31]
- Rates of PFS were also similar in both groups. In view of its low morbidity and mortality, it was concluded that radiation therapy should be considered the preferred locoregional treatment for these patients.[31]
- In the largest series (EORTC-08941), 579 patients with histologically- or cytologically-proven stage IIIA (N2) NSCLC were given three cycles of platinum-based induction chemotherapy.[31] The 333 responding patients were subsequently randomly assigned to surgical resection or radiation therapy. Of the 154 patients (92%) who underwent surgery, 50% had a radical resection, 42% had a pathological downstaging, and 5% had a pathological complete response; 4% died after surgery. Postoperative (adjuvant) radiation therapy (PORT) was administered to 62 patients (40%) in the surgery arm. Among the 154 patients (93%) who received radiation therapy, overall compliance to the radiation therapy prescription was 55%, and grade 3 to 4 acute and late esophageal and pulmonary toxic effects occurred in 4% and 7% of patients; one patient died of radiation pneumonitis.
Adjuvant radiation therapy
The value of PORT has been assessed.[27] Although some studies suggest that PORT can improve local control for node-positive patients whose tumors were resected, it remains controversial whether it can improve survival. The optimal dose of thoracic PORT is not known at this time. Most studies cited used doses ranging from 30 Gy to 60 Gy, typically provided in 2 Gy to 2.5 Gy fractions.[27]
As referred to in the National Cancer Institute of Canada (NCIC) Clinical Trials Group JBR.10 study (NCT00002583), PORT may be considered in selected patients to reduce the risk of local recurrence, if any of the following are present:[23]
- Involvement of multiple nodal stations.
- Extracapsular tumor spread.
- Close or microscopically positive resection margins.
Evidence (adjuvant radiation therapy):
Evidence from one large meta-analysis, subset analyses of randomized trials, and one large population study suggest that PORT may reduce local recurrence. Results from these studies on the effect of PORT on OS are conflicting.
- A meta-analysis of ten randomized trials that evaluated PORT versus surgery alone showed the following:
- No difference in OS for the entire PORT group or for the subset of N2 patients.[18][Level of evidence A1]
- Results from a nonrandomized subanalysis of the ANITA trial, comparing 5-year OS in N2 patients who did or did not receive PORT, found the following:[6]
- Higher survival rates in patients who received radiation therapy in the observation arm (21% in patients who received PORT vs. 17% in patients who did not receive PORT) and in the chemotherapy arm (47% with PORT vs. 34% without PORT); however, statistical tests of comparison were not conducted.[6]
- Results from the Surveillance, Epidemiology, and End Results (SEER) Program [28] suggest the following:
- The large SEER retrospective study (N = 7,465) found superior survival rates associated with radiation therapy in N2 disease (HR, 0.855; 95% CI, 0.762–0.959).
There is benefit of PORT in stage IIIA (N2) disease, and the role of PORT in early stages of NSCLC should be clarified in ongoing phase III trials. Further analysis is needed to determine whether these outcomes can be modified with technical improvements, better definitions of target volumes, and limitation of cardiac volume in the radiation portals.[18]
Treatment Options for Unresectable Stage IIIA NSCLC
Treatment options for patients with unresectable stage IIIA NSCLC include:
Chemoradiation therapy
The addition of sequential and concurrent chemotherapy to radiation therapy has been evaluated in prospective randomized trials and meta-analyses. Overall, concurrent treatment may provide the greatest benefit in survival with an increase in toxic effects.
Concomitant platinum-based radiation chemotherapy may improve survival of patients with locally advanced NSCLC. However, the available data are insufficient to accurately define the size of such a potential treatment benefit and the optimal schedule of chemotherapy.[32]
Evidence (chemoradiation therapy):
- A meta-analysis of patient data from 11 randomized clinical trials showed the following:[33]
- Cisplatin-based combinations plus radiation therapy resulted in a 10% reduction in the risk of death compared with radiation therapy alone.[33][Level of evidence A1]
- A meta-analysis of 13 trials (based on 2,214 evaluable patients) showed the following:[34]
- The addition of concurrent chemotherapy to radical radiation therapy reduced the risk of death at 2 years (relative risk [RR], 0.93; 95% CI, 0.88–0.98; P = .01).
- For the 11 trials with platinum-based chemotherapy, RR was 0.93 (95% CI, 0.87–0.99; P = .02).[34]
- A meta-analysis of individual data from 1,764 patients was based on nine trials and showed the following:[32]
- The HRdeath among patients treated with radiation therapy and chemotherapy compared with radiation therapy alone was 0.89 (95% CI, 0.81–0.98; P = .02), corresponding to an absolute benefit of chemotherapy of 4% at 2 years.
- The combination of platinum with etoposide appeared to be more effective than platinum alone.
Concurrent versus sequential chemoradiation therapy
The results from two randomized trials (including RTOG-9410 [NCT01134861]) and a meta-analysis indicate that concurrent chemotherapy and radiation therapy may provide greater survival benefit, albeit with more toxic effects, than sequential chemotherapy and radiation therapy.[35,36,37][Level of evidence A1]
Evidence (concurrent vs. sequential chemoradiation therapy):
- In the first trial, the combination of mitomycin C, vindesine, and cisplatin were given concurrently with split-course daily radiation therapy to 56 Gy compared with chemotherapy followed by continuous daily radiation therapy to 56 Gy.[35]
- Five-year OS rates favored concurrent therapy (27% vs. 9%).
- Myelosuppression was greater among patients in the concurrent arm, but treatment-related mortality was less than 1% in both arms.[35]
- In the second trial, 610 patients were randomly assigned to sequential chemotherapy with cisplatin and vinblastine followed by 63 Gy of radiation therapy, concurrent chemoradiation therapy using the same regimen, or concurrent chemotherapy with cisplatin and etoposide with twice-daily radiation therapy.[37]
- Median and 5-year survival were superior in the concurrent chemotherapy with daily radiation therapy arm (17 months vs. 14.6 months and 16% vs. 10% for sequential regimen [P = .046]).[37]
- Two smaller studies also reported OS results that favored concurrent over sequential chemotherapy and radiation, although the results did not reach statistical significance.[36,38][Level of evidence A1]
- A meta-analysis of three trials evaluated concurrent versus sequential treatment (711 patients).[34]
- The analysis indicated a significant benefit of concurrent over sequential treatment (RR, 0.86; 95% CI, 0.78–0.95; P = .003). All studies used cisplatin-based regimens and once-daily radiation therapy.[34]
- More deaths (3% OS rate) were reported in the concurrent arm, but this did not reach statistical significance (RR, 1.60; CI, 0.75–3.44; P = .2).
- There was more acute esophagitis (grade 3 or worse) with concurrent treatment (range, 17%–26%) compared with sequential treatment (range, 0%–4%; RR, 6.77; P = .001). Overall, the incidence of neutropenia (grade 3 or worse) was similar in both arms.
Radiation therapy dose escalation for concurrent chemoradiation
With improvement in radiation therapy–delivery technology in the 1990s, including tumor-motion management and image guidance, phase I/II trials demonstrated the feasibility of dose-escalation radiation therapy to 74 Gy with concurrent chemotherapy.[39,40,41] However, a phase III trial of a conventional dose of 60 Gy versus dose escalation to 74 Gy with concurrent weekly carboplatin/paclitaxel did not demonstrate improved local control or PFS, and OS was worse with dose escalation (HR, 1.38; 95% CI, 1.09–1.76; P = .004). There was a nonsignificant increase in grade 5 events with dose escalation (10% vs. 2%) and higher incidence of grade 3 esophagitis (21% vs. 7%; P = .0003). Thus, there is no clear benefit in radiation dose escalation beyond 60 Gy for stage III NSCLC.[42][Level of evidence A1]
Consolidation therapy following concurrent chemoradiation
Evidence (consolidation therapy following concurrent chemoradiation):
- The randomized phase III PROCLAIM study [NCT00686959] enrolled 598 patients with newly diagnosed, stage IIIA/B, unresectable, nonsquamous NSCLC.[43] Patients were randomly assigned on a 1:1 ratio to either of two arms:
- Arm A: Pemetrexed (500 mg/m2) and cisplatin (75 mg/m2) intravenously every 3 weeks for three cycles plus concurrent thoracic radiation therapy (60 to 66 Gy) followed by pemetrexed consolidation every 3 weeks for four cycles.
- Arm B: Standard therapy with etoposide (50 mg/m2) and cisplatin (50 mg/m2) intravenously every 4 weeks for two cycles plus concurrent thoracic radiation therapy (60 to 66 Gy) followed by two cycles of consolidation platinum-based doublet chemotherapy.
The primary objective was OS. The study was designed as a superiority trial with 80% power to detect an OS HR of 0.74 with a type 1 error of .05. This study randomly assigned 598 patients (arm A, 301; arm B, 297) and treated 555 patients (arm A, 283; arm B, 272).
- Enrollment was stopped early because of futility.
- OS in arm A was not superior to arm B (HR, 0.98; 95% CI, 0.79–1.20; median, 26.8 vs. 25.0 months; P = .831).
- Arm A had a significantly lower incidence of any drug-related grade 3 to 4 adverse events (64.0% vs. 76.8%; P = .001), including neutropenia (24.4% vs. 44.5%; P < .001), during the overall treatment period.
Consolidation immunotherapy
Durvalumab
Durvalumab is a selective human IgG1 monoclonal antibody that blocks PD-L1 binding to programmed death 1 (PD-1) and CD80, allowing T cells to recognize and kill tumor cells.[44]
Evidence (durvalumab following concurrent chemoradiation):
- The phase III PACIFIC trial (NCT02125461) enrolled 713 patients with stage III NSCLC whose disease had not progressed after two or more cycles of platinum-based chemoradiation therapy. Patients were randomly assigned in a 2:1 ratio to receive durvalumab (10 mg/kg intravenously) or placebo (every 2 weeks for up to 12 months).[44]
- At a median follow-up of 34.2 months for all patients and 61.6 months for censored patients, the median OS was 47.5 months for all patients and 29.1 months for censored patients (stratified HR, 0.72; 95% CI, 0.59–0.89). The median PFS was 16.9 months in the durvalumab group and 5.6 months in the placebo group (stratified HR, 0.55; 95% CI, 0.45–0.68).
- The estimated 5-year OS rates were 42.9% (95% CI, 38.2%–47.4%) in the durvalumab group and 33.4% (95% CI, 27.3%–39.6%) in the placebo group.[45][Level of evidence A1]
- The estimated 5-year PFS rates were 33.1% (95% CI, 28.0%–38.2%) in the durvalumab group and 19% (95% CI, 13.6%–25.2%) in the placebo group.
- Grade 3 or 4 adverse events occurred in 29.9% of patients treated with durvalumab and in 26.1% of patients treated with placebo. The most common adverse event of grade 3 or 4 was pneumonia in 4.4% of the patients who received durvalumab and in 3.8% of the patients who received placebo.
Osimertinib (for patients withEGFRmutations)
Evidence (osimertinib following concurrent chemoradiation therapy):
- The phase III, double-blind, placebo-controlled LAURA trial (NCT03521154) included patients with unresectable EGFR-mutated stage III NSCLC who had not progressed during or after definitive chemoradiation therapy. A total of 216 patients who had undergone chemoradiation therapy were randomly assigned to receive either osimertinib (n = 143) or placebo (n = 73). The primary end point was PFS as assessed by blinded independent central review.[46]
- The median PFS was 39.1 months with osimertinib and 5.6 months with placebo (HRdisease progression or death, 0.16; 95% CI, 0.10–0.24; P < .001).
- At 36 months, the OS rate was 84% for patients in the osimertinib group (95% CI, 75%–89%) and 74% for patients in the placebo group (95% CI, 57%–85%) (HRdeath, 0.81; 95% CI, 0.42–1.56; P = .53) (at 20% data maturity).[46][Level of evidence A1]
- Grade 3 or higher adverse events occurred in 35% of patients in the osimertinib group and 12% of patients in the placebo group. Radiation pneumonitis was reported in 48% of patients in the osimertinib group and 38% of patients in the placebo group.
Other systemic consolidation therapies
The addition of induction chemotherapy before concurrent chemotherapy and radiation therapy has not been shown to improve survival.[47][Level of evidence A1]
Randomized trials of other consolidation systemic therapies, including docetaxel,[48] gefitinib,[49] and tecemotide (MUC1 antigen-specific immunotherapy) [50] have not shown an improvement in OS.[Level of evidence A1]
Radiation therapy
Locally advanced unresectable tumors
Radiation therapy alone may provide benefit to patients with locally advanced unresectable stage IIIA NSCLC.
Radiation therapy with traditional dose and fractionation schedules (1.8–2.0 Gy per fraction per day to 60–70 Gy in 6–7 weeks) results in reproducible long-term survival benefit in 5% to 10% of patients and significant palliation of symptoms.[51]
Evidence (radiation therapy for locally advanced unresectable tumor):
- One prospective randomized clinical study showed the following:[52]
- Radiation therapy given continuously (including weekends) as three daily fractions (continuous hyperfractionated accelerated radiation therapy) improved OS compared with radiation therapy given as one daily fraction.[52][Level of evidence A1]
- Patterns of failure for patients treated with radiation therapy alone included both locoregional and distant failures.
Although patients with unresectable stage IIIA disease may benefit from radiation therapy, long-term outcomes have generally been poor because of local and systemic relapse.
Palliative treatment
Radiation therapy may be effective in palliating symptomatic local involvement with NSCLC, such as:
- Tracheal, esophageal, or bronchial compression.
- Pain.
- Vocal cord paralysis.
- Hemoptysis.
- Superior vena cava syndrome.
In some cases, endobronchial laser therapy and/or brachytherapy has been used to alleviate proximal obstructing lesions.[53]
Evidence (radiation therapy for palliative treatment):
- A systematic review identified six randomized trials of high-dose rate endobronchial brachytherapy (HDREB) alone or with external-beam radiation therapy (EBRT) or laser therapy.[54]
- Better overall symptom palliation and fewer re-treatments were required in previously untreated patients using EBRT alone.[54][Level of evidence A3]
- Although EBRT is frequently prescribed for symptom palliation, there is no consensus about when the fractionation scheme should be used.
- For EBRT, different multifraction regimens appear to provide similar symptom relief;[55,56,57,58,59,60] however, single-fraction radiation therapy may be insufficient for symptom relief compared with hypofractionated or standard regimens, as seen in the NCIC Clinical Trials Group trial (NCT00003685).[57][Level of evidence A3]
- Evidence of a modest increase in survival in patients with better performance status given high-dose EBRT is available.[55,56][Level of evidence A1]
- HDREB provided palliation of symptomatic patients with recurrent endobronchial obstruction previously treated by EBRT, when it was technically feasible.
Treatment Options for Superior Sulcus Tumors
Treatment options for superior sulcus tumors include:
NSCLC of the superior sulcus, frequently termed Pancoast tumors, occurs in less than 5% of patients.[61,62] Superior sulcus tumors usually arise from the apex of the lung and are challenging to treat because of their proximity to structures at the thoracic inlet. At this location, tumors may invade the parietal pleura, chest wall, brachial plexus, subclavian vessels, stellate ganglion, and adjacent vertebral bodies. However, Pancoast tumors are amenable to curative treatment, especially in patients with T3, N0 disease.
Adverse prognostic factors include the presence of mediastinal nodal metastases (N2 disease), spine or subclavian-vessel involvement (T4 disease), and limited resection (R1 or R2).
Surgery
Evidence (surgery):
- Retrospective case series have reported that complete resection was achieved in only 64% of T3, N0 tumors and 39% of T4, N0 tumors.[63]
Chemoradiation therapy followed by surgery
Evidence (chemoradiation therapy):
- Two large, prospective, multicenter phase II trials have evaluated induction chemoradiation therapy followed by resection.[64,65]
- In the first trial (NCT00002642), 110 eligible patients were enrolled with mediastinoscopy negative, clinical T3–4, N0–1 tumors of the superior sulcus.[65] Induction treatment was two cycles of etoposide and cisplatin with 45 Gy of concurrent radiation therapy.
- The induction regimen was well tolerated, and only five participants had grade 3 or higher toxic effects.
- Induction chemoradiation therapy could sterilize the primary lesion. Induction therapy was completed by 104 patients (95%). Of the 95 patients eligible for surgery, 88 (80%) underwent thoracotomy, two (1.8%) died postoperatively, and 83 (76%) had complete resections.
- Pathological complete response or minimal microscopic disease was seen in 61 (56%) resection specimens. Pathological complete response led to better survival than when any residual disease was present (P = .02).
- Five-year survival was 44% for all patients and 54% after complete resection, with no difference between T3 and T4 tumors. Disease progression occurred mainly in distant sites.
- In the second trial, 75 patients were enrolled and treated with induction therapy with mitomycin C, vindesine, and cisplatin combined with 45 Gy of radiation therapy.[64] Fifty-seven patients (76%) underwent surgical resection, and complete resection was achieved in 51 patients (68%).
- There were 12 patients with pathological complete response.
- Major postoperative morbidity, including chylothorax, empyema, pneumonitis, adult respiratory distress syndrome, and bleeding, was observed in eight patients. There were three treatment-related deaths.
- At 3 years, the DFS rate was 49%, and the OS rate was 61%; at 5 years, the DFS rate was 45%, and the OS rate was 56%.[64][Level of evidence C2]
- In the first trial (NCT00002642), 110 eligible patients were enrolled with mediastinoscopy negative, clinical T3–4, N0–1 tumors of the superior sulcus.[65] Induction treatment was two cycles of etoposide and cisplatin with 45 Gy of concurrent radiation therapy.
Radiation therapy dose escalation for concurrent chemoradiation
With improvement in radiation therapy–delivery technology in the 1990s, including tumor-motion management and image guidance, phase I/II trials demonstrated the feasibility of dose-escalation radiation therapy to 74 Gy with concurrent chemotherapy.[39,40,41] However, a phase III trial of a conventional dose of 60 Gy versus dose escalation to 74 Gy with concurrent weekly carboplatin/paclitaxel did not demonstrate improved local control or PFS, and OS was worse with dose escalation (HR, 1.38 [1.09–1.76]; P = .004). There was a nonsignificant increase in grade 5 events with dose escalation (10% vs. 2%) and higher incidence of grade 3 esophagitis (21% vs. 7%; P = .0003). Thus, there is no clear benefit in radiation dose escalation beyond 60 Gy for stage III NSCLC.[42][Level of evidence A1]
Radiation therapy alone
While radiation therapy is an integral part of the treatment of Pancoast tumors, variations in dose, treatment technique, and staging that were used in various published series make it difficult to determine its effectiveness.[61,62]
Small, retrospective series of radiation therapy in patients who were only clinically staged have reported 5-year survival rates of 0% to 40%, depending on T stage, total radiation dose, and other prognostic factors. Induction radiation therapy and en bloc resection was shown to be potentially curative.
Evidence (radiation therapy):
- In the preoperative setting, a dose of 45 Gy over 5 weeks is generally recommended, while a dose of approximately 61 Gy is required when using definitive radiation therapy as the primary modality.[61,62]
Treatment Options for Tumors That Invade the Chest Wall
Treatment options for tumors that invade the chest wall include:
- Surgery.
- Surgery and radiation therapy.
- Radiation therapy alone.
- Chemotherapy combined with radiation therapy and/or surgery.
Selected patients with bulky primary tumors that directly invade the chest wall can obtain long-term survival with surgical management provided that their tumor is completely resected.
Evidence (radical surgery):
- In a small case series of 97 patients, the 5-year survival rate of patients who had completely resected T3, N0, M0 disease was 44.2%. For patients with completely resected T3, N1, M0 disease, the 5-year survival rate was 40.0%. In patients with completely resected T3, N2, M0 disease, the 5-year survival rate was 6.2%.[66][Level of evidence C2]
- In a small case series of 104 patients, the 5-year survival rate of patients who had completely resected T3, N0, M0 disease was 67.3%. For patients with completely resected T3, N1, M0 disease, the 5-year survival rate was 100.0%. In patients with completely resected T3, N2, M0 disease, the 5-year survival rate was 17.9%.[67][Level of evidence C2]
- In a case series of 309 patients treated at three centers, patients who underwent en bloc resection had superior outcomes compared with patients who underwent extrapleural resections (60.3% vs. 39.1%; P = .03).[68][Level of evidence C2]
Adjuvant chemotherapy is recommended, and radiation therapy is reserved for cases with unclear resection margins. Survival rates were lower in patients who underwent incomplete resection and had mediastinal lymph node involvement. Combined-modality approaches have been evaluated to improve ability to achieve complete resection.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Darling GE, Allen MS, Decker PA, et al.: Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 141 (3): 662-70, 2011.
- Albain KS, Swann RS, Rusch VW, et al.: Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 374 (9687): 379-86, 2009.
- Manser R, Wright G, Hart D, et al.: Surgery for early stage non-small cell lung cancer. Cochrane Database Syst Rev (1): CD004699, 2005.
- Allen MS, Darling GE, Pechet TT, et al.: Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 81 (3): 1013-9; discussion 1019-20, 2006.
- Burdett SS, Stewart LA, Rydzewska L: Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. Cochrane Database Syst Rev (3): CD006157, 2007.
- Douillard JY, Rosell R, De Lena M, et al.: Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 7 (9): 719-27, 2006.
- Gilligan D, Nicolson M, Smith I, et al.: Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 369 (9577): 1929-37, 2007.
- Albain KS, Rusch VW, Crowley JJ, et al.: Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 13 (8): 1880-92, 1995.
- Suntharalingam M, Paulus R, Edelman MJ, et al.: Radiation therapy oncology group protocol 02-29: a phase II trial of neoadjuvant therapy with concurrent chemotherapy and full-dose radiation therapy followed by surgical resection and consolidative therapy for locally advanced non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 84 (2): 456-63, 2012.
- Forde PM, Spicer J, Lu S, et al.: Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 386 (21): 1973-1985, 2022.
- Provencio M, Serna-Blasco R, Nadal E, et al.: Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J Clin Oncol 40 (25): 2924-2933, 2022.
- Wakelee H, Liberman M, Kato T, et al.: Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 389 (6): 491-503, 2023.
- Heymach JV, Harpole D, Mitsudomi T, et al.: Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J Med 389 (18): 1672-1684, 2023.
- Cascone T, Awad MM, Spicer JD, et al.: CheckMate 77T: Phase III study comparing neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) vs neoadjuvant placebo plus chemo followed by surgery and adjuvant NIVO or placebo for previously untreated, resectable stage II–IIIb NSCLC. [Abstract] Ann Oncol 34 (Suppl 2): A-LBA1, S1295, 2023.
- Lu S, Zhang W, Wu L, et al.: Perioperative Toripalimab Plus Chemotherapy for Patients With Resectable Non-Small Cell Lung Cancer: The Neotorch Randomized Clinical Trial. JAMA 331 (3): 201-211, 2024.
- Pignon JP, Tribodet H, Scagliotti GV, et al.: Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 26 (21): 3552-9, 2008.
- Winton T, Livingston R, Johnson D, et al.: Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352 (25): 2589-97, 2005.
- Arriagada R, Bergman B, Dunant A, et al.: Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350 (4): 351-60, 2004.
- Scagliotti GV, Fossati R, Torri V, et al.: Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst 95 (19): 1453-61, 2003.
- Hotta K, Matsuo K, Ueoka H, et al.: Role of adjuvant chemotherapy in patients with resected non-small-cell lung cancer: reappraisal with a meta-analysis of randomized controlled trials. J Clin Oncol 22 (19): 3860-7, 2004.
- Edell ES, Cortese DA: Photodynamic therapy in the management of early superficial squamous cell carcinoma as an alternative to surgical resection. Chest 102 (5): 1319-22, 1992.
- Corti L, Toniolo L, Boso C, et al.: Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med 39 (5): 394-402, 2007.
- Pepe C, Hasan B, Winton TL, et al.: Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol 25 (12): 1553-61, 2007.
- Tsuboi M, Herbst RS, John T, et al.: Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N Engl J Med 389 (2): 137-147, 2023.
- O'Brien M, Paz-Ares L, Marreaud S, et al.: Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 23 (10): 1274-1286, 2022.
- Felip E, Altorki N, Zhou C, et al.: Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398 (10308): 1344-1357, 2021.
- PORT Meta-analysis Trialists Group: Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev (2): CD002142, 2005.
- Lally BE, Zelterman D, Colasanto JM, et al.: Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol 24 (19): 2998-3006, 2006.
- Johnstone DW, Byhardt RW, Ettinger D, et al.: Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 54 (2): 365-9, 2002.
- Taylor NA, Liao ZX, Cox JD, et al.: Equivalent outcome of patients with clinical Stage IIIA non-small-cell lung cancer treated with concurrent chemoradiation compared with induction chemotherapy followed by surgical resection. Int J Radiat Oncol Biol Phys 58 (1): 204-12, 2004.
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al.: Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 99 (6): 442-50, 2007.
- Aupérin A, Le Péchoux C, Pignon JP, et al.: Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 17 (3): 473-83, 2006.
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 311 (7010): 899-909, 1995.
- Rowell NP, O'rourke NP: Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev (4): CD002140, 2004.
- Furuse K, Fukuoka M, Kawahara M, et al.: Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 17 (9): 2692-9, 1999.
- Fournel P, Robinet G, Thomas P, et al.: Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol 23 (25): 5910-7, 2005.
- Curran WJ, Paulus R, Langer CJ, et al.: Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 103 (19): 1452-60, 2011.
- Zatloukal P, Petruzelka L, Zemanova M, et al.: Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer 46 (1): 87-98, 2004.
- Rosenman JG, Halle JS, Socinski MA, et al.: High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys 54 (2): 348-56, 2002.
- Socinski MA, Blackstock AW, Bogart JA, et al.: Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol 26 (15): 2457-63, 2008.
- Bradley JD, Bae K, Graham MV, et al.: Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol 28 (14): 2475-80, 2010.
- Bradley JD, Paulus R, Komaki R, et al.: Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 16 (2): 187-99, 2015.
- Senan S, Brade A, Wang LH, et al.: PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 34 (9): 953-62, 2016.
- Antonia SJ, Villegas A, Daniel D, et al.: Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 377 (20): 1919-1929, 2017.
- Spigel DR, Faivre-Finn C, Gray JE, et al.: Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 40 (12): 1301-1311, 2022.
- Lu S, Kato T, Dong X, et al.: Osimertinib after Chemoradiotherapy in Stage III EGFR-Mutated NSCLC. N Engl J Med 391 (7): 585-597, 2024.
- Vokes EE, Herndon JE, Kelley MJ, et al.: Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 25 (13): 1698-704, 2007.
- Hanna N, Neubauer M, Yiannoutsos C, et al.: Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 26 (35): 5755-60, 2008.
- Kelly K, Chansky K, Gaspar LE, et al.: Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 26 (15): 2450-6, 2008.
- Butts C, Socinski MA, Mitchell PL, et al.: Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 15 (1): 59-68, 2014.
- Komaki R, Cox JD, Hartz AJ, et al.: Characteristics of long-term survivors after treatment for inoperable carcinoma of the lung. Am J Clin Oncol 8 (5): 362-70, 1985.
- Saunders M, Dische S, Barrett A, et al.: Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet 350 (9072): 161-5, 1997.
- Miller JI, Phillips TW: Neodymium:YAG laser and brachytherapy in the management of inoperable bronchogenic carcinoma. Ann Thorac Surg 50 (2): 190-5; discussion 195-6, 1990.
- Ung YC, Yu E, Falkson C, et al.: The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small-cell lung cancer: a systematic review. Brachytherapy 5 (3): 189-202, 2006 Jul-Sep.
- Sundstrøm S, Bremnes R, Aasebø U, et al.: Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol 22 (5): 801-10, 2004.
- Lester JF, Macbeth FR, Toy E, et al.: Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev (4): CD002143, 2006.
- Bezjak A, Dixon P, Brundage M, et al.: Randomized phase III trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (NCIC CTG SC.15). Int J Radiat Oncol Biol Phys 54 (3): 719-28, 2002.
- Erridge SC, Gaze MN, Price A, et al.: Symptom control and quality of life in people with lung cancer: a randomised trial of two palliative radiotherapy fractionation schedules. Clin Oncol (R Coll Radiol) 17 (1): 61-7, 2005.
- Kramer GW, Wanders SL, Noordijk EM, et al.: Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol 23 (13): 2962-70, 2005.
- Senkus-Konefka E, Dziadziuszko R, Bednaruk-Młyński E, et al.: A prospective, randomised study to compare two palliative radiotherapy schedules for non-small-cell lung cancer (NSCLC). Br J Cancer 92 (6): 1038-45, 2005.
- Rusch VW: Management of Pancoast tumours. Lancet Oncol 7 (12): 997-1005, 2006.
- Narayan S, Thomas CR: Multimodality therapy for Pancoast tumor. Nat Clin Pract Oncol 3 (9): 484-91, 2006.
- Rusch VW, Parekh KR, Leon L, et al.: Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J Thorac Cardiovasc Surg 119 (6): 1147-53, 2000.
- Kunitoh H, Kato H, Tsuboi M, et al.: Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol 26 (4): 644-9, 2008.
- Rusch VW, Giroux DJ, Kraut MJ, et al.: Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 25 (3): 313-8, 2007.
- Matsuoka H, Nishio W, Okada M, et al.: Resection of chest wall invasion in patients with non-small cell lung cancer. Eur J Cardiothorac Surg 26 (6): 1200-4, 2004.
- Facciolo F, Cardillo G, Lopergolo M, et al.: Chest wall invasion in non-small cell lung carcinoma: a rationale for en bloc resection. J Thorac Cardiovasc Surg 121 (4): 649-56, 2001.
- Doddoli C, D'Journo B, Le Pimpec-Barthes F, et al.: Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg 80 (6): 2032-40, 2005.
Treatment of Stages IIIB and IIIC NSCLC
On the basis of the Surveillance, Epidemiology, and End Results (SEER) Program registry, the estimated incidence of stage IIIB non-small cell lung cancer (NSCLC) is 17.6%.[1] The anticipated 5-year survival rate for most patients who present with clinical stage IIIB NSCLC is 3% to 7%.[2] In small case series, selected patients with T4, N0–1 disease, solely as the result of satellite tumor nodule(s) within the primary lobe, had 5-year survival rates of 20%.[3,4][Level of evidence C1]
Treatment Options for Stages IIIB and IIIC NSCLC
Treatment options for stages IIIB NSCLC and IIIC NSCLC include:
- Sequential or concurrent chemotherapy and radiation therapy.
- Radiation therapy alone.
- New fractionation schedules (under clinical evaluation).
- Radiosensitizers (NCT02186847) (under clinical evaluation).
- Combined-modality approaches (under clinical evaluation).
- Incorporation of targeted agents into combined modality therapy in patients with EGFR-mutant or ALK-translocated tumors (RTOG-1306 [NCT01822496]; 11-464 [NCT01553942]) (under clinical evaluation).
- Adaptive radiation therapy using positron emission tomography–based response assessment during treatment (RTOG-1106/ACRIN-6697) (under clinical evaluation).
In general, patients with stages IIIB and IIIC NSCLC do not benefit from surgery alone and are best managed by initial chemotherapy, chemotherapy plus radiation therapy, or radiation therapy alone, depending on:
- Sites of tumor involvement.
- The patient's performance status.
Most patients with excellent performance status are candidates for combined-modality chemotherapy and radiation therapy with the following exceptions:
- Selected patients with T4, N0 disease may be treated with combined-modality therapy and surgery similar to patients with superior sulcus tumors.
Patients with stages IIIB or IIIC NSCLC are candidates for clinical trials, which may lead to improvement in the control of disease.
Sequential or concurrent chemotherapy and radiation therapy
Many randomized studies of patients with unresectable stage III NSCLC show that treatment with preoperative or concurrent cisplatin-based chemotherapy and radiation therapy to the chest is associated with improved survival compared with treatment that uses radiation therapy alone. Although patients with unresectable stages IIIB or IIIC disease may benefit from radiation therapy, long-term outcomes have generally been poor, often the result of local and systemic relapse. The addition of sequential and concurrent chemotherapy to radiation therapy has been evaluated in prospective randomized trials.
Evidence (sequential or concurrent chemotherapy and radiation therapy):
- A meta-analysis of patient data from 11 randomized clinical trials showed the following:[5]
- Cisplatin-based combinations plus radiation therapy resulted in a 10% reduction in the risk of death compared with radiation therapy alone.[5][Level of evidence A1]
- A meta-analysis of 13 trials (based on 2,214 evaluable patients) showed the following:[6]
- The addition of concurrent chemotherapy to radical radiation therapy reduced the risk of death at 2 years (relative risk [RR], 0.93; 95% confidence interval [CI], 0.88–0.98; P = .01).
- For the 11 trials with platinum-based chemotherapy, RR was 0.93 (95% CI, 0.87–0.99; P = .02).[6]
- A meta-analysis of individual data from 1,764 patients evaluated nine trials.[7]
- The hazard ratio (HR)death among patients treated with radiation therapy and chemotherapy compared with radiation therapy alone was 0.89 (95% CI, 0.81–0.98; P = .02) corresponding to an absolute benefit of chemotherapy of 4% at 2 years.
- The combination of platinum with etoposide appeared to be more effective than platinum alone. Concomitant platinum-based chemotherapy and radiation therapy may improve survival of patients with locally advanced NSCLC. However, the available data are insufficient to accurately define the size of such a potential treatment benefit and the optimal schedule of chemotherapy.[7]
- The results from two randomized trials (including RTOG-9410 [NCT01134861]) and a meta-analysis indicate that concurrent chemotherapy and radiation therapy provide greater survival benefit, albeit with more toxic effects, than sequential chemotherapy and radiation therapy.[8,9,10][Level of evidence A1]
- In the first trial, the combination of mitomycin C, vindesine, and cisplatin were given concurrently with split-course daily radiation therapy to 56 Gy compared with chemotherapy followed by continuous daily radiation therapy to 56 Gy.[8]
- Five-year overall survival (OS) rates favored concurrent therapy (27% vs. 9%).
- Myelosuppression was greater among patients in the concurrent arm, but treatment-related mortality was less than 1% in both arms.[8]
- In the second trial, 610 patients were randomly assigned to sequential chemotherapy with cisplatin and vinblastine followed by 63 Gy of radiation therapy, concurrent chemoradiation therapy using the same regimen, or concurrent chemotherapy with cisplatin and etoposide with twice-daily radiation therapy.[9,10]
- Median and 5-year survival were superior in the concurrent chemotherapy with daily radiation therapy arm (17 months vs. 14.6 months and 16% vs. 10% for sequential regimen [P = .046]).
- Two smaller studies also reported OS results that favored concurrent over sequential chemotherapy and radiation, although the results did not reach statistical significance.[10][Level of evidence A1]; [11]
- In the first trial, the combination of mitomycin C, vindesine, and cisplatin were given concurrently with split-course daily radiation therapy to 56 Gy compared with chemotherapy followed by continuous daily radiation therapy to 56 Gy.[8]
- A meta-analysis of three trials evaluated concurrent versus sequential treatment (711 patients).[6]
- The analysis indicated a significant benefit of concurrent versus sequential treatment (RR, 0.86; 95% CI, 0.78–0.95; P = .003). All used cisplatin-based regimens and once-daily radiation therapy.[6]
- More deaths (3% overall) were reported in the concurrent arm, but this did not reach statistical significance (RR, 1.60; CI, 0.75–3.44; P = .2).
- There was more acute esophagitis (grade 3 or worse) with concurrent treatment (range, 17%–26%) compared with sequential treatment (range, 0%–4%; RR, 6.77; P = .001). Overall, the incidence of neutropenia (grade 3 or worse) was similar in both arms.
Radiation therapy dose escalation for concurrent chemoradiation
With improvement in radiation therapy–delivery technology in the 1990s, including tumor-motion management and image guidance, phase I/II trials demonstrated the feasibility of dose-escalation radiation therapy to 74 Gy with concurrent chemotherapy.[12,13,14] However, a phase III trial of a conventional dose of 60 Gy versus dose escalation to 74 Gy with concurrent weekly carboplatin/paclitaxel did not demonstrate improved local control or progression-free survival (PFS), and OS was worse with dose escalation (HR, 1.38 [1.09–1.76]; P = .004). There was a nonsignificant increase in grade 5 events with dose escalation (10% vs. 2%) and higher incidence of grade 3 esophagitis (21% vs. 7%; P = .0003).[15][Level of evidence A1]
Systemic consolidation therapy before or after concurrent chemoradiation therapy
Consolidation immunotherapy
Durvalumab
Durvalumab is a selective human IgG1 monoclonal antibody that blocks programmed death-ligand 1 (PD-L1) binding to programmed death 1 (PD-1) and CD80, allowing T cells to recognize and kill tumor cells.[16]
Evidence (durvalumab):
- The phase III PACIFIC trial (NCT02125461) enrolled 713 patients with stage III NSCLC whose disease had not progressed after two or more cycles of platinum-based chemoradiation therapy. Patients were randomly assigned in a 2:1 ratio to receive durvalumab (10 mg/kg intravenously) or placebo (every 2 weeks for up to 12 months).[16] The coprimary end points were PFS assessed by blinded independent central review and OS (unplanned for the interim analysis).
- At the interim analysis, the coprimary end point of PFS was met. The median PFS was 16.8 months with durvalumab versus 5.6 months with placebo (HR, 0.52; 95% CI, 0.42–0.65; P < .001).[16][Level of evidence B1] The 18-month PFS rate was 44.2% with durvalumab versus 27% with placebo.
- PFS benefit was seen across all prespecified subgroups and was irrespective of PD-L1 expression before chemoradiation therapy or smoking status. EGFR mutations were observed in 6% of patients (29 treated with durvalumab vs. 14 treated with placebo). The unstratified HR for the EGFR-mutated subgroup was 0.76 (95% CI, 0.35–1.64).
- Grade 3 or 4 adverse events occurred in 29.9% of patients treated with durvalumab and in 26.1% of patients treated with placebo. The most common adverse event of grade 3 or 4 was pneumonia in 4.4% of patients treated with durvalumab and in 3.8% of patients treated with placebo.
- OS was not assessed at the interim analysis.
Osimertinib (for patients withEGFRmutations)
Evidence (osimertinib following concurrent chemoradiation therapy):
- The phase III, double-blind, placebo-controlled LAURA trial (NCT03521154) included patients with unresectable EGFR-mutated stage III NSCLC who had not progressed during or after definitive chemoradiation therapy. A total of 216 patients who had undergone chemoradiation therapy were randomly assigned to receive either osimertinib (n = 143) or placebo (n = 73).The primary end point was PFS as assessed by blinded independent central review.[17]
- The median PFS was 39.1 months with osimertinib and 5.6 months with placebo (HRdisease progression or death, 0.16; 95% CI, 0.10–0.24; P < .001).
- At 36 months, the OS rate was 84% for patients in the osimertinib group (95% CI, 75%–89%) and 74% for patients in the placebo group (95% CI, 57%–85%) (HRdeath, 0.81; 95% CI, 0.42–1.56; P = .53) (at 20% data maturity).[17][Level of evidence A1]
- Grade 3 or higher adverse events occurred in 35% of patients in the osimertinib group and 12% of patients in the placebo group. Radiation pneumonitis was reported in 48% of patients in the osimertinib group and 38% of patients in the placebo group.
Other systemic consolidation therapies
The addition of induction chemotherapy before concurrent chemotherapy and radiation therapy has not been shown to improve survival.[18][Level of evidence A1]
Randomized trials of other consolidation systemic therapies, including docetaxel,[19] gefitinib,[20] and tecemotide (MUC1 antigen-specific immunotherapy) [21] have not shown an improvement in OS.[Level of evidence A1]
The role of consolidation systemic therapy after concurrent chemotherapy and radiation therapy for unresectable NSCLC remains unclear. Phase III trials of consolidation systemic therapy including conventional chemotherapy (docetaxel),[19] tyrosine kinase inhibitors (gefitinib),[20] and immunotherapy (tecemotide: MUC1 antigen-specific immunotherapy) [21] have not shown an improvement in OS.[Level of evidence A1]
Radiation therapy alone
For treatment of locally advanced unresectable tumor in patients who are not candidates for chemotherapy
Radiation therapy alone may provide benefit to patients with locally advanced unresectable stage III NSCLC.
Radiation therapy with traditional dose and fractionation schedules (1.8–2.0 Gy per fraction per day to 60–70 Gy in 6–7 weeks) results in reproducible long-term survival benefit in 5% to 10% of patients and significant palliation of symptoms.[22]
Evidence (radiation therapy for locally advanced unresectable tumor):
- One prospective randomized clinical study showed the following:
- Radiation therapy given as three daily fractions improved OS compared with radiation therapy given as one daily fraction.[23][Level of evidence A1]
- Patterns of failure for patients treated with radiation therapy alone included both locoregional and distant failures.
For patients requiring palliative treatment
Radiation therapy may be effective in palliating symptomatic local involvement with NSCLC, such as:
- Tracheal, esophageal, or bronchial compression.
- Pain.
- Vocal cord paralysis.
- Hemoptysis.
- Superior vena cava syndrome.
In some cases, endobronchial laser therapy and/or brachytherapy has been used to alleviate proximal obstructing lesions.[24]
Evidence (radiation therapy for palliative treatment):
- A systematic review identified six randomized trials of high-dose rate endobronchial brachytherapy (HDREB) alone or with external-beam radiation therapy (EBRT) or laser therapy.[25]
- Better overall symptom palliation and fewer re-treatments were required in previously untreated patients using EBRT alone.[25][Level of evidence A3]
- HDREB provided palliation of symptomatic patients with recurrent endobronchial obstruction previously treated by EBRT, when it was technically feasible.
- Although EBRT is frequently prescribed for symptom palliation, there is no consensus about when the fractionation scheme should be used.
- Although different multifraction regimens appear to provide similar symptom relief,[26,27,28,29,30,31] single-fraction radiation may be insufficient for symptom relief compared with hypofractionated or standard regimens, as shown in the National Cancer Institute of Canada Clinical Trials Group trial (NCT00003685).[28][Level of evidence A3]
- Evidence of a modest increase in survival in patients with better performance status given high-dose radiation therapy is available.[26,27][Level of evidence A1]
Patients with stages IIIB or IIIC disease with poor performance status are candidates for chest radiation therapy to palliate pulmonary symptoms (e.g., cough, shortness of breath, hemoptysis, or pain).[22][Level of evidence C1] For more information, see Cardiopulmonary Syndromes and Cancer Pain.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Wisnivesky JP, Yankelevitz D, Henschke CI: Stage of lung cancer in relation to its size: part 2. Evidence. Chest 127 (4): 1136-9, 2005.
- Mountain CF: Revisions in the International System for Staging Lung Cancer. Chest 111 (6): 1710-7, 1997.
- Deslauriers J, Brisson J, Cartier R, et al.: Carcinoma of the lung. Evaluation of satellite nodules as a factor influencing prognosis after resection. J Thorac Cardiovasc Surg 97 (4): 504-12, 1989.
- Urschel JD, Urschel DM, Anderson TM, et al.: Prognostic implications of pulmonary satellite nodules: are the 1997 staging revisions appropriate? Lung Cancer 21 (2): 83-7; discussion 89-91, 1998.
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 311 (7010): 899-909, 1995.
- Rowell NP, O'rourke NP: Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev (4): CD002140, 2004.
- Aupérin A, Le Péchoux C, Pignon JP, et al.: Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 17 (3): 473-83, 2006.
- Furuse K, Fukuoka M, Kawahara M, et al.: Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 17 (9): 2692-9, 1999.
- Curran WJ, Paulus R, Langer CJ, et al.: Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 103 (19): 1452-60, 2011.
- Fournel P, Robinet G, Thomas P, et al.: Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol 23 (25): 5910-7, 2005.
- Zatloukal P, Petruzelka L, Zemanova M, et al.: Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer 46 (1): 87-98, 2004.
- Rosenman JG, Halle JS, Socinski MA, et al.: High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys 54 (2): 348-56, 2002.
- Socinski MA, Blackstock AW, Bogart JA, et al.: Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol 26 (15): 2457-63, 2008.
- Bradley JD, Bae K, Graham MV, et al.: Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol 28 (14): 2475-80, 2010.
- Bradley JD, Paulus R, Komaki R, et al.: Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 16 (2): 187-99, 2015.
- Antonia SJ, Villegas A, Daniel D, et al.: Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 377 (20): 1919-1929, 2017.
- Lu S, Kato T, Dong X, et al.: Osimertinib after Chemoradiotherapy in Stage III EGFR-Mutated NSCLC. N Engl J Med 391 (7): 585-597, 2024.
- Vokes EE, Herndon JE, Kelley MJ, et al.: Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 25 (13): 1698-704, 2007.
- Hanna N, Neubauer M, Yiannoutsos C, et al.: Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 26 (35): 5755-60, 2008.
- Kelly K, Chansky K, Gaspar LE, et al.: Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 26 (15): 2450-6, 2008.
- Butts C, Socinski MA, Mitchell PL, et al.: Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 15 (1): 59-68, 2014.
- Langendijk JA, ten Velde GP, Aaronson NK, et al.: Quality of life after palliative radiotherapy in non-small cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys 47 (1): 149-55, 2000.
- Komaki R, Cox JD, Hartz AJ, et al.: Characteristics of long-term survivors after treatment for inoperable carcinoma of the lung. Am J Clin Oncol 8 (5): 362-70, 1985.
- Miller JI, Phillips TW: Neodymium:YAG laser and brachytherapy in the management of inoperable bronchogenic carcinoma. Ann Thorac Surg 50 (2): 190-5; discussion 195-6, 1990.
- Ung YC, Yu E, Falkson C, et al.: The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small-cell lung cancer: a systematic review. Brachytherapy 5 (3): 189-202, 2006 Jul-Sep.
- Sundstrøm S, Bremnes R, Aasebø U, et al.: Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol 22 (5): 801-10, 2004.
- Lester JF, Macbeth FR, Toy E, et al.: Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev (4): CD002143, 2006.
- Bezjak A, Dixon P, Brundage M, et al.: Randomized phase III trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (NCIC CTG SC.15). Int J Radiat Oncol Biol Phys 54 (3): 719-28, 2002.
- Erridge SC, Gaze MN, Price A, et al.: Symptom control and quality of life in people with lung cancer: a randomised trial of two palliative radiotherapy fractionation schedules. Clin Oncol (R Coll Radiol) 17 (1): 61-7, 2005.
- Kramer GW, Wanders SL, Noordijk EM, et al.: Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol 23 (13): 2962-70, 2005.
- Senkus-Konefka E, Dziadziuszko R, Bednaruk-Młyński E, et al.: A prospective, randomised study to compare two palliative radiotherapy schedules for non-small-cell lung cancer (NSCLC). Br J Cancer 92 (6): 1038-45, 2005.
Treatment of Newly Diagnosed Stage IV, Relapsed, and Recurrent NSCLC
Factors Affecting Treatment Selection
Forty percent of patients with newly diagnosed non-small cell lung cancer (NSCLC) have stage IV disease. Treatment goals are to prolong survival and control disease-related symptoms. Treatment options include cytotoxic chemotherapy, targeted agents, and immunotherapy. Factors influencing treatment selection include comorbidity, performance status, histology, and molecular and immunologic features of the cancer. Therefore, assessment of tumor-genomic changes and programmed death-ligand 1 (PD-L1) expression is critical before initiating therapy. Radiation therapy and surgery are generally used in selective cases for symptom palliation.
Factors that affect selection of treatment include:
History and molecular features
Patients with nonsquamous cell histology, good performance status, no history of hemoptysis or other bleeding, or recent history of cardiovascular events may benefit from the addition of bevacizumab to paclitaxel and carboplatin. Patients with tumors harboring sensitizing mutations in exons 19 or 21 of EGFR, particularly those from East Asia, never smokers, and those with adenocarcinoma may benefit from epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) as an alternative to first- or second-line chemotherapy. Patients with tumors harboring ALK translocations, ROS1 rearrangements, or NTRK fusions may benefit from anaplastic lymphoma kinase (ALK), ROS1, or neurotrophic tyrosine kinase (NTRK) inhibitors as an alternative to first- or second-line chemotherapy.
Patients with tumors expressing PD-L1 (>50% by immunohistochemistry) have improved survival with pembrolizumab. The addition of pembrolizumab to carboplatin-plus-pemetrexed chemotherapy for nonsquamous advanced lung cancer improves survival irrespective of PD-L1 expression.[1][Level of evidence A1] For patients with stage IV or recurrent NSCLC and PD-L1 expression on at least 1% of tumor cells, frontline combination immunotherapy with nivolumab and ipilimumab increases overall survival (OS).[2][Level of evidence A1] Second-line systemic therapy with nivolumab, docetaxel, pemetrexed, or pembrolizumab for PD-L1−positive tumors also improves survival in patients with good performance status (who have not received the same or a similar agent in the first-line setting).[3][Level of evidence A1]
The role of systemic therapy in patients with an Eastern Cooperative Oncology Group (ECOG) performance status below 2 is less certain.
Patients with adenocarcinoma may benefit from pemetrexed [4] and bevacizumab, as well as from combination chemotherapy with pembrolizumab. Patients with unresectable, locally advanced or metastatic, well-differentiated, nonfunctional, neuroendocrine tumors benefit from the mammalian target of rapamycin (mTOR) inhibitor, everolimus.
Age and comorbidity
Evidence supports the concept that older patients with good performance status and limited comorbidity may benefit from combination chemotherapy. Age alone should not dictate treatment-related decisions in patients with advanced NSCLC. Older patients with a good performance status enjoy longer survival and a better quality of life when treated with chemotherapy compared with supportive care alone. Caution should be exercised when extrapolating data for patients aged 70 to 79 years to patients aged 80 years or older because only a very small number of patients aged 80 years or older have been enrolled in clinical trials, and the benefit in this group is uncertain.[5,6]
Evidence (age and comorbidity):
- Platinum-containing combination chemotherapy regimens provide clinical benefit when compared with supportive care or single-agent therapy; however, such treatment may be contraindicated in some older patients because of the age-related reduction in the functional reserve of many organs and/or comorbid conditions. Approximately two-thirds of patients with NSCLC are aged 65 years or older, and approximately 40% are aged 70 years or older.[7] Surveillance, Epidemiology, and End Results (SEER) Program data suggest that the percentage of patients older than 70 years is closer to 50%.
- A review of the SEER Medicare data from 1994 to 1999 found a much lower rate of chemotherapy use than expected for the overall population.[8] The same data suggested that older patients may have more comorbidities or a higher rate of functional compromise that would make study participation difficult, if not contraindicated; lack of clinical trial data may influence decisions to treat individual patients with standard chemotherapy.
- Single-agent chemotherapy and combination chemotherapy clearly benefit at least some older patients. In the Elderly Lung Cancer Vinorelbine Italian Study, 154 patients who were older than 70 years were randomly assigned to vinorelbine or supportive care.[9]
- Patients who were treated with vinorelbine had a 1-year survival rate of 32%, compared with 14% for those who were treated with supportive care alone. Quality-of-life parameters were also significantly improved in the chemotherapy arm, and toxic effects were acceptable.
- A trial from Japan compared single-agent docetaxel with vinorelbine in 180 patients older than 70 years with good performance status.[10]
- Response rates (22% vs. 10%) and progression-free survival (PFS) (5.4 months vs. 3.1 months) were significantly better with docetaxel, but median survival (14.3 months vs. 9.9 months) and 1-year survival rates (59% vs. 37%) did not reach statistical significance.
- Retrospective data analyzing and comparing younger (age <70 years) patients with older (age ≥70 years) patients who participated in large randomized trials of doublet combinations have also shown that older patients may derive the same survival benefit, but with a higher risk of toxic effects in the bone marrow.[5,6,11,12,13,14]
Performance status
Performance status is among the most important prognostic factors for survival of patients with NSCLC.[15] The benefit of therapy for this group of patients has been evaluated through retrospective analyses and prospective clinical trials.
The results support further evaluation of chemotherapeutic approaches for both metastatic and locally advanced NSCLC; however, the efficacy of current platinum-based chemotherapy combinations is such that no specific regimen can be regarded as standard therapy. Outside of a clinical trial setting, chemotherapy should only be given to patients with good performance status and evaluable tumor lesions, who desire this treatment after being fully informed of its anticipated risks and limited benefits.
Randomized controlled trials of patients with stage IV disease and good performance status have shown that cisplatin-based chemotherapy improves survival and palliates disease-related symptoms.[3][Level of evidence A1]
Evidence (performance status):
- The Cancer and Leukemia Group B trial (CLB-9730 [NCT00003117]), which compared carboplatin and paclitaxel with single-agent paclitaxel, enrolled 99 patients with a performance status of 2 (18% of the study's population).[13]
- When compared with patients with a performance status of 0 to 1, who had a median survival of 8.8 months and a 1-year survival rate of 38%, the corresponding median survival figures for patients with a performance status of 2 were 3.0 months and a 1-year survival rate of 14%; this demonstrates the poor prognosis conferred by a lower performance status. These differences were statistically significant.
- When patients with a performance status of 2 were analyzed by treatment arm, those who received combination chemotherapy had a significantly higher response rate (24% vs. 10%), longer median survival (4.7 months vs. 2.4 months), and a superior 1-year survival rate (18% vs. 10%), compared with those who were treated with single-agent paclitaxel.[13]
- A phase III trial compared single-agent pemetrexed with the combination of carboplatin and pemetrexed in 205 patients with a performance status of 2 who had not had any previous chemotherapy.[16][Level of evidence A1]
- Median OS was 5.3 months for the pemetrexed-alone group and 9.3 months for the carboplatin-and-pemetrexed group (hazard ratio [HR], 0.62; 95% confidence interval [CI], 0.46–0.83; P = .001).
- Median PFS was 2.8 months for the pemetrexed-alone group and 5.8 months for the carboplatin-and-pemetrexed group (P < .001).
- The response rates were 10.3% for the pemetrexed-alone group and 23.8% for the carboplatin-and-pemetrexed group (P = .032).
- Side effects were more frequent in the combination arm, as expected.
This study, which was performed in eight centers in Brazil and one center in the United States, reported rates of OS and PFS that were higher than has historically been noted in most, although not all, other published studies. This may indicate differences in patient selection.
- A subset analysis of 68 patients with a performance status of 2 from a trial that randomly assigned more than 1,200 patients to four platinum-based regimens has been published.
- Despite a high incidence of adverse events, including five deaths, the final analysis showed that the overall toxic effects experienced by patients with a performance status of 2 was not significantly different from that experienced by patients with a performance status of 0 to 1.
- An efficacy analysis demonstrated an overall response rate of 14%, median survival time of 4.1 months, and a 1-year survival rate of 19%; all were substantially inferior to the patients with performance status of 0 to 1.
- A phase II randomized trial (E-1599 [NCT00006004]) of attenuated dosages of cisplatin plus gemcitabine and carboplatin plus paclitaxel included 102 patients with a performance status of 2.[17]
- Response rates were 25% in the cisplatin-plus-gemcitabine arm and 16% in the carboplatin-plus-paclitaxel arm; median survival times were 6.8 months in the cisplatin-plus-gemcitabine arm and 6.1 months in the carboplatin-plus-paclitaxel arm; 1-year survival rates were 25% in the cisplatin-plus-gemcitabine arm and 19% in the carboplatin-plus-paclitaxel arm. None of these differences was statistically significant, but the survival figures were longer than expected, based on historical controls.
- Results from two trials suggest that patients with a performance status of 2 may experience symptom improvement.[18,19]
Treatment Options for Newly Diagnosed Stage IV, Relapsed, and Recurrent NSCLC (First-Line Therapy)
Treatment options for patients with newly diagnosed stage IV, relapsed, and recurrent disease include:
- Cytotoxic combination chemotherapy with platinum (cisplatin or carboplatin) and paclitaxel, gemcitabine, docetaxel, vinorelbine, irinotecan, protein-bound paclitaxel, or pemetrexed.
- Combination chemotherapy with monoclonal antibodies.
- Maintenance therapy after first-line chemotherapy (for patients with stable or responding disease after four cycles of platinum-based combination chemotherapy).
- Maintenance therapy following first-line chemotherapy.
- Pemetrexed following first-line platinum-based combination chemotherapy.
- Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) with or without chemotherapy (for patients with EGFR mutations).
- EGFR-directed therapy (for patients with EGFR exon 20 insertion mutations).
- Anaplastic lymphoma kinase (ALK) inhibitors (for patients with ALK translocations).
- BRAF V600E and MEK inhibitors (for patients with BRAF V600E mutations).
- ROS1 inhibitors (for patients with ROS1 rearrangements).
- Neurotrophic tyrosine kinase (NTRK) inhibitors (for patients with NTRK fusions).
- RET inhibitors (for patients with RET fusions).
- MET inhibitors (for patients with MET exon 14 skipping mutations).
- Immune checkpoint inhibitors with or without chemotherapy.
- mTOR inhibitors (for patients with unresectable, locally advanced or metastatic, progressive, well-differentiated, nonfunctional, neuroendocrine tumors).
- Local therapies and special considerations.
- Endobronchial laser therapy and/or brachytherapy (for obstructing lesions).[20]
- External-beam radiation therapy (EBRT) (primarily for palliation of local symptomatic tumor growth).[21,22,23]
- Treatment of second primary tumor.
- Treatment of brain metastases.
- Clinical trials can be considered as first-line therapy.
Cytotoxic combination chemotherapy
Combination chemotherapy
The type and number of chemotherapy drugs to be used for the treatment of patients with advanced NSCLC has been extensively evaluated in randomized controlled trials and meta-analyses.
Several randomized trials have evaluated various drugs combined with either cisplatin or carboplatin in previously untreated patients with advanced NSCLC. On the basis of meta-analyses of the trials, the following conclusions can be drawn:
- Certain three-drug combinations that add so-called targeted agents may result in superior survival.
- EGFR inhibitors may benefit selected patients with EGFR mutations.
- Maintenance chemotherapy after four cycles of platinum combination chemotherapy may improve PFS and OS.
- Platinum combinations with vinorelbine, paclitaxel, docetaxel, gemcitabine, irinotecan, protein-bound paclitaxel, and pemetrexed yield similar improvements in survival. Types and frequencies of toxic effects differ, and these may determine the preferred regimen for an individual patient. Patients with adenocarcinoma may benefit from pemetrexed.
- Cisplatin and carboplatin yield similar improvements in outcome with different toxic effects. Some, but not all, trials and meta-analyses of trials suggest that outcomes with cisplatin may be superior, although with a higher risk of certain toxicities such as nausea and vomiting.
- Nonplatinum combinations offer no advantage to platinum-based chemotherapy, and some studies demonstrate inferiority.
- Three-drug combinations of the commonly used chemotherapy drugs do not result in superior survival and are more toxic than two-drug combinations.
Evidence (combination chemotherapy):
- The Cochrane Collaboration reviewed data from all randomized controlled trials published between January 1980 and June 2006, comparing a doublet regimen with a single-agent regimen or comparing a triplet regimen with a doublet regimen in patients with advanced NSCLC.[24] Sixty-five trials (13,601 patients) were identified.
- In the trials that compared a doublet regimen with a single-agent regimen, a significant increase was observed in tumor response (odds ratio [OR], 0.42; 95% CI, 0.37–0.47; P < .001) and 1-year survival (OR, 0.80; 95% CI, 0.70–0.91; P < .001) in favor of the doublet regimen. The absolute benefit in 1-year survival was 5%, which corresponds to an increase in 1-year survival from 30% with a single-agent regimen to 35% with a doublet regimen. The rates of grades 3 and 4 toxic effects caused by doublet regimens were statistically increased compared with rates after single-agent therapy, with ORs ranging from 1.2 to 6.2. Infection rates did not increase in doublet regimens.
- There was no increase in 1-year survival (OR, 1.01; 95% CI, 0.85–1.21; P = .88) for triplet regimens versus doublet regimens. The median survival ratio was 1.00 (95% CI, 0.94–1.06; P = .97).
- Several meta-analyses have evaluated whether cisplatin or carboplatin regimens are superior, with variable results.[25,26,27] One meta-analysis reported individual patient data for 2,968 patients entered in nine randomized trials.[25]
- The objective response rate was higher for patients treated with cisplatin (30%) than for patients treated with carboplatin (24%); (OR, 1.37; 95% CI, 1.16–1.61; P < .001).
- Carboplatin treatment was associated with a nonstatistically significant increase in the hazard of mortality relative to treatment with cisplatin (HR, 1.07; 95% CI, 0.99–1.15; P = .100).
- In patients with nonsquamous cell tumors and in patients treated with third-generation chemotherapy, carboplatin-based chemotherapy was associated with a statistically significant increase in mortality (HR, 1.12; 95% CI, 1.01–1.23 in patients with nonsquamous cell tumors and HR, 1.11; 95% CI, 1.01–1.21 in patients treated with third-generation chemotherapy).
- Treatment-related toxic effects were also assessed in the meta-analysis. More thrombocytopenia was seen with carboplatin than with cisplatin (12% vs. 6%; OR, 2.27; 95% CI, 1.71–3.01; P < .001), but cisplatin caused more nausea and vomiting (8% vs. 18%; OR, 0.42; 95% CI, 0.33–0.53; P < .001) and renal toxic effects (0.5% vs. 1.5%; OR, 0.37; 95% CI, 0.15–0.88; P = .018).
- The authors concluded that treatment with cisplatin was not associated with a substantial increase in the overall risk of severe toxic effects. This comprehensive individual-patient meta-analysis is consistent with the conclusions of other meta-analyses that were based on essentially the same clinical trials, but which used only published data.
- Three literature-based meta-analyses have trials that compared platinum with nonplatinum combinations.[28,29,30]
- The first meta-analysis identified 37 assessable trials that included 7,633 patients.[28]
- A 62% increase in the OR for response was attributable to platinum-based therapy (OR, 1.62; 95% CI, 1.46–1.8; P < .001). The 1-year survival rate was increased by 5% with platinum-based regimens (34% vs. 29%; OR, 1.21; 95% CI, 1.09–1.35; P = .003).
- No statistically significant increase in 1-year survival was found when platinum therapies were compared with third-generation-based combination regimens (OR, 1.11; 95% CI, 0.96–1.28; P = .17).
- The toxic effects of platinum-based regimens was significantly higher for hematologic toxic effects, nephrotoxic effects, and nausea and vomiting but not for neurological toxic effects, febrile neutropenia rate, or toxic death rate. These results are consistent with the second literature-based meta-analysis.
- The second meta-analysis identified 17 trials that included 4,920 patients.[29]
- The use of platinum-based doublet regimens was associated with a slightly higher survival at 1 year (relative risk [RR], 1.08; 95% CI, 1.01%–1.16%; P = .03) and a better partial response (RR, 1.11; 95% CI, 1.02–1.21; P = .02), with a higher risk of anemia, nausea, and neurological toxic effects.
- In subanalyses, cisplatin-based doublet regimens improved survival at 1 year (RR, 1.16%; 95% CI, 1.06–1.27; P = .001), complete response (RR, 2.29; 95% CI, 1.08–4.88; P = .03), and partial response (RR, 1.19; 95% CI, 1.07–1.32; P = .002), with an increased risk of anemia, neutropenia, neurological toxic effects, and nausea.
- Conversely, carboplatin-based doublet regimens did not increase survival at 1 year (RR, 0.95; 95% CI, 0.85–1.07; P = .43).
- The third meta-analysis of phase III trials randomizing platinum-based versus nonplatinum combinations as first-line chemotherapy identified 14 trials.[30] Experimental arms were gemcitabine and vinorelbine (n = 4), gemcitabine and taxane (n = 7), gemcitabine and epirubicin (n = 1), paclitaxel and vinorelbine (n = 1), and gemcitabine and ifosfamide (n = 1). This meta-analysis was limited to the set of 11 phase III studies that used a platinum-based doublet (2,298 patients in the platinum-based arm and 2,304 patients in the nonplatinum arm).
- Patients treated with a platinum-based regimen benefited from a statistically significant reduction in the risk of death at 1 year (OR, 0.88; 95% CI, 0.78–0.99; P = .044) and a lower risk of being refractory to chemotherapy (OR, 0.87; CI, 0.73–0.99; P = .049).
- Forty-four (1.9%) toxic-related deaths were reported for platinum-based regimens and 29 (1.3%) toxic-related deaths were reported for nonplatinum regimens (OR, 1.53; CI, 0.96–2.49; P = .08). An increased risk of grade 3 to 4 gastrointestinal and hematologic toxic effects for patients treated with platinum-based chemotherapy was statistically demonstrated. There was no statistically significant increase in the risk of febrile neutropenia (OR, 1.23; CI, 0.94–1.60; P = .063).
- The first meta-analysis identified 37 assessable trials that included 7,633 patients.[28]
Drug and dose schedule
Among the active combinations, definitive recommendations regarding drug dose and schedule cannot be made, except for carboplatin, pemetrexed, and pembrolizumab for patients with nonsquamous tumor histology.
Evidence (drug and dose schedule):
- One meta-analysis of seven trials that included 2,867 patients assessed the benefit of docetaxel versus vinorelbine.[31] Docetaxel was administered with a platinum agent in three trials, with gemcitabine in two trials, or as monotherapy in two trials. Vinca alkaloid (vinorelbine in six trials and vindesine in one trial) was administered with cisplatin in six trials or alone in one trial.
- The pooled estimate for OS showed an 11% improvement in favor of docetaxel (HR, 0.89; 95% CI, 0.82–0.96; P = .004). Sensitivity analyses that considered only vinorelbine as a comparator or only the doublet regimens showed similar improvements.
- Grade 3 to 4 neutropenia and grade 3 to 4 serious adverse events were less frequent with docetaxel-based regimens (OR, 0.59; 95% CI, 0.38–0.89; P = .013) versus vinca alkaloid-based regimens (OR, 0.68; 95% CI, 0.55–0.84; P < .001).
- Two randomized trials compared weekly versus every-3-week dosing of paclitaxel and carboplatin, which reported no significant difference in efficacy and better tolerability for weekly administration.[32,33] Although meta-analyses of randomized controlled trials suggest that cisplatin combinations may be superior to carboplatin or nonplatinum combinations, the clinical relevance of the differences in efficacy must be balanced against the anticipated tolerability, logistics of administration, and familiarity of the medical staff in making treatment decisions for individual patients.
- A large, noninferiority, phase III randomized study compared the OS in 1,725 chemotherapy-naïve patients with stage IIIB/IV NSCLC and a performance status of 0 to 1.[4] Patients received cisplatin 75 mg/m2 on day 1 and gemcitabine 1,250 mg/m2 on days 1 and 8 (n = 863) or cisplatin 75 mg/m2 and pemetrexed 500 mg/m2 on day 1 (n = 862) every 3 weeks for up to six cycles.
- OS for cisplatin and pemetrexed (median survival, 10.3 months) was noninferior to cisplatin and gemcitabine (median survival, 10.3 months; HR, 0.94; 95% CI, 0.84%–1.05%).
- In patients with adenocarcinoma (n = 847), OS was statistically superior for cisplatin and pemetrexed (12.6 months) versus cisplatin and gemcitabine (10.9 months); in patients with large cell carcinoma (n = 153), OS was statistically superior for cisplatin and pemetrexed (10.4 months) versus cisplatin and gemcitabine (6.7 months).
- In contrast, in patients with squamous cell histology (n = 473), there was a significant improvement in survival with cisplatin and gemcitabine (10.8 months) versus cisplatin and pemetrexed (9.4 months). For cisplatin and pemetrexed, rates of grade 3 or 4 neutropenia, anemia, and thrombocytopenia (P ≤ .001); febrile neutropenia (P = .002); and alopecia (P < .001) were significantly lower, whereas grade 3 or 4 nausea (P = .004) was more common.
- The results of this study suggested that the cisplatin and pemetrexed doublet is another alternative doublet for first-line chemotherapy for advanced NSCLC. The results also suggested that there may be differences in outcome depending on histology.
Combination chemotherapy with monoclonal antibodies
Bevacizumab
Evidence (bevacizumab):
- Two randomized trials have evaluated the addition of bevacizumab, an antibody targeting vascular endothelial growth factor, to standard first-line combination chemotherapy.
- In a randomized study of 878 patients with recurrent or advanced stage IIIB/IV NSCLC, 444 patients received paclitaxel and carboplatin alone, and 434 patients received paclitaxel and carboplatin plus bevacizumab.[34] Chemotherapy was administered every 3 weeks for six cycles, and bevacizumab was administered every 3 weeks until disease progression was evident or toxic effects were intolerable. Patients with squamous cell tumors, brain metastases, clinically significant hemoptysis, or inadequate organ function or performance status (ECOG performance status >1) were excluded.
- Median survival was 12.3 months in the group assigned to chemotherapy plus bevacizumab, as compared with 10.3 months in the chemotherapy-alone group (HRdeath, 0.79; P = .003).
- Median PFS was 6.2 months in the group assigned to chemotherapy plus bevacizumab (HRdisease progression, 0.66; P < .001), with a 35% response rate (P < .001), and 4.5 months in the chemotherapy-alone group (HRdisease progression, 0.66; P < .001), with a 15% response rate (P < .001).
- Rates of clinically significant bleeding were 4.4% in the group assigned to chemotherapy plus bevacizumab and 0.7% in the chemotherapy-alone group (P < .001). There were 15 treatment-related deaths in the chemotherapy-plus-bevacizumab group, including five from pulmonary hemorrhage.
- For this subgroup of patients with NSCLC, the addition of bevacizumab to paclitaxel and carboplatin may provide survival benefit.[34][Level of evidence A1]
- Another randomized, phase III trial investigated the efficacy and safety of cisplatin-gemcitabine plus bevacizumab.[35] Patients were randomly assigned to receive cisplatin (80 mg/m2) and gemcitabine (1,250 mg/m2) for up to six cycles, plus low-dose bevacizumab (7.5 mg/kg), high-dose bevacizumab (15 mg/kg), or placebo every 3 weeks until disease progression. The primary end point was amended from OS to PFS during the course of the study. A total of 1,043 patients were accrued (placebo group, n = 347; low-dose group, n = 345; high-dose group, n = 351).
- PFS was significantly prolonged with the addition of bevacizumab; the HRs for PFS were 0.75 in the low-dose group (median PFS, 6.7 months vs. 6.1 months for the placebo group; P = .03) and 0.82 in the high-dose group compared with the placebo group (median PFS, 6.5 months vs. 6.1 months for the placebo group; P = .03).[35][Level of evidence A1]
- Objective response rates were also improved with the addition of bevacizumab, and they were 20.1% for placebo, 34.1% for low-dose bevacizumab, and 30.4% for high-dose bevacizumab plus cisplatin/gemcitabine.
- Incidence of grade 3 or greater adverse events was similar across arms.
- Grade 3 or greater pulmonary hemorrhage rates were 1.5% or less for all arms, despite 9% of patients receiving therapeutic anticoagulation.
- These results support the addition of bevacizumab to platinum-containing chemotherapy, but the results are far less impressive than when the carboplatin-paclitaxel combination was used.
- Furthermore, no significant difference in survival was shown in this study, as reported in abstract form.
- Altogether, these findings may suggest that the backbone of chemotherapy may be important when bevacizumab is added.
- In a randomized study of 878 patients with recurrent or advanced stage IIIB/IV NSCLC, 444 patients received paclitaxel and carboplatin alone, and 434 patients received paclitaxel and carboplatin plus bevacizumab.[34] Chemotherapy was administered every 3 weeks for six cycles, and bevacizumab was administered every 3 weeks until disease progression was evident or toxic effects were intolerable. Patients with squamous cell tumors, brain metastases, clinically significant hemoptysis, or inadequate organ function or performance status (ECOG performance status >1) were excluded.
Cetuximab
Evidence (cetuximab):
- Two trials have evaluated the addition of cetuximab to first-line combination chemotherapy.[36,37]
- In the first trial, 676 chemotherapy-naïve patients with stage IIIB (pleural effusion) or stage IV NSCLC, without restrictions by histology or EGFR expression, received cetuximab with taxane (paclitaxel or docetaxel with carboplatin) or combination chemotherapy.[36]
- The addition of cetuximab did not result in a statistically significant improvement in PFS, the primary study end point, or OS.
- Median PFS was 4.40 months for patients in the cetuximab-chemotherapy arm versus 4.24 months for patients in the taxane-carboplatin arm (HR, 0.902; 95% CI, 0.761–1.069; P = .236).
- Median OS was 9.69 months for patients in the cetuximab-chemotherapy arm versus 8.38 months for patients in the chemotherapy-alone arm (HR, 0.890; 95% CI, 0.754–1.051; P = .169).
- No significant associations were found between EGFR expression, EGFR mutation, EGFR copy number, or KRAS mutations and PFS, OS, and response in the treatment-specific analyses.[38]
- The second trial was composed of 1,125 chemotherapy-naïve patients with advanced EGFR-expressing stage IIIB/IV NSCLC treated with cisplatin-vinorelbine chemotherapy plus cetuximab or chemotherapy alone.[37]
- The primary study end point, OS, was longer for patients treated with cetuximab and chemotherapy (median 11.3 months vs. 10.1 months; HRdeath, 0.871; 95% CI, 0.762–0.996; P = .044).
- A survival benefit was seen in all histological subgroups; however, survival benefit was not seen in non-White or Asian patients. Only the interaction between the treatment and the ethnic origin was significant (P = .011).
- The main cetuximab-related adverse event was acne-like rash (grade 3, 10%).
- It is not clear whether the differences in outcome in these two studies are the result of differences in the study populations, tumor characterization for EGFR expression, or chemotherapy regimens.
- In the first trial, 676 chemotherapy-naïve patients with stage IIIB (pleural effusion) or stage IV NSCLC, without restrictions by histology or EGFR expression, received cetuximab with taxane (paclitaxel or docetaxel with carboplatin) or combination chemotherapy.[36]
Necitumumab
Evidence (necitumumab):
- Two phase III trials have evaluated the addition of the second-generation, recombinant, human immunoglobulin G1 EGFR antibody, necitumumab, to platinum-doublet chemotherapy in the first-line treatment of patients with advanced nonsquamous cell and squamous cell NSCLC.[39,40]
- The SQUIRE trial (NCT00981058) randomly assigned 1,093 patients with advanced squamous NSCLC to receive either first-line chemotherapy with cisplatin and gemcitabine or the same regimen with the addition of necitumumab (800 mg on day 1 and day 8 of each cycle).[40]
- Median OS was prolonged with the addition of necitumumab (11.5 months vs. 9.9 months; P = .01).
- PFS was also prolonged with the addition of necitumumab (5.7 months vs. 5.5 months); however, the overall response rate was similar in both groups (31% vs. 28%).
- Grades 3 and 4 adverse events were higher in the necitumumab-containing arm (72% vs. 62%).
- Necitumumab is associated with higher toxicity and relatively modest benefit.
- The INSPIRE trial (NCT00982111) randomly assigned 633 patients with advanced nonsquamous NSCLC to receive either first-line chemotherapy with cisplatin and pemetrexed or to cisplatin and pemetrexed with the addition of necitumumab (800 mg on day 1 and day 8 of each cycle).[39]
- This study showed no benefit from the addition of necitumumab to standard first-line chemotherapy for advanced nonsquamous NSCLC.
- OS was 11.3 months (95% CI, 9.5–13.4) for patients in the necitumumab-containing arm versus 11.5 months (95% CI, 10.1–13.1) for patients in the chemotherapy alone arm; P = .96. Similarly, there was no difference between the arms in terms of objective response or PFS.
- Serious adverse events and rates of grades 3 and 4 adverse events, including thromboembolic events, were higher in patients in the necitumumab-containing arm; the incidence of treatment-related deaths was also higher (5% vs. 3%).
- On the basis of these results, necitumumab is not recommended as combination therapy with standard first-line chemotherapy for patients with advanced nonsquamous NSCLC.
- The SQUIRE trial (NCT00981058) randomly assigned 1,093 patients with advanced squamous NSCLC to receive either first-line chemotherapy with cisplatin and gemcitabine or the same regimen with the addition of necitumumab (800 mg on day 1 and day 8 of each cycle).[40]
Maintenance therapy after first-line chemotherapy (for patients with stable or responding disease after four cycles of platinum-based combination chemotherapy)
One extensively investigated treatment strategy in NSCLC is maintenance therapy after initial response to chemotherapy. Options for maintenance therapy that have been investigated include:
- Continuing the initial combination chemotherapy regimen.
- Continuing only single-agent chemotherapy.
- Introducing a new agent as maintenance.
Multiple randomized trials have evaluated the efficacy of continuing first-line combination cytotoxic chemotherapy beyond three to four cycles.
Evidence (maintenance therapy following first-line chemotherapy):
- None of the trials of continued cytotoxic combinations showed a significant OS advantage with additional or longer durations beyond four cycles. For patients with nonsquamous NSCLC, two studies have demonstrated improved PFS and OS with either switch or continuous maintenance chemotherapy (e.g., maintenance pemetrexed after initial cisplatin and gemcitabine or maintenance pemetrexed after initial cisplatin and pemetrexed).[41]
- Three trials found statistically significantly improved PFS or time to progression with additional chemotherapy.[42,43,44]
- No consistent improvement in quality of life was reported.[43,45,46]
- Chemotherapy-related toxicities were greater with prolonged chemotherapy.[45,46]
These data suggest that PFS and OS for patients with nonsquamous NSCLC may be improved either by continuing an effective chemotherapy beyond four cycles or by immediate initiation of alternative chemotherapy. The improvement in PFS, however, is tempered by an increase in adverse events including additional cytotoxic chemotherapy and no consistent improvement in quality of life. For patients who have stable disease or who respond to first-line therapy, evidence does not support the continuation of combination cytotoxic chemotherapy until disease progression or the initiation of a different chemotherapy before disease progression. Collectively, these trials suggest that first-line cytotoxic combination chemotherapy should be stopped at disease progression or after four cycles in patients whose disease is not responding to treatment; it can be administered for no more than six cycles.[42,43,45,46] For patients with nonsquamous NSCLC who have a response or stable disease after four to six cycles of platinum combination chemotherapy, maintenance chemotherapy with pemetrexed should be considered.[41]
Evidence (first-line platinum-based combination chemotherapy followed by pemetrexed):
The findings of two randomized trials (NCT00102804 and NCT00789373) have shown improved outcomes with the addition of pemetrexed after standard first-line platinum-based combination chemotherapy.[44,47]
- In the first trial, 663 patients with stage IIIB/IV disease who had not progressed on four cycles of nonpemetrexed platinum–based chemotherapy were randomly assigned (in a 2:1 ratio) to receive pemetrexed or placebo until disease progression.[47]
- Both the primary end point of PFS and the secondary end point of OS were statistically significantly prolonged with the addition of maintenance pemetrexed (median PFS, 4.3 months vs. 2.6 months; HR, 0.50; 95% CI, 0.42–0.61; P < .0001; median OS, 13.4 months vs. 10.6 months; HR, 0.79; 95% CI, 0.65–0.95; P = .012).
- Benefit was not seen in patients with squamous histology.
- Higher than grade 3 toxicity and treatment discontinuations that resulted from drug-related toxic effects were higher in the pemetrexed group than in the placebo group.
- No pemetrexed-related deaths occurred.
- Relatively fewer patients in the pemetrexed group than in the placebo group received systemic postdiscontinuation therapy (227 [51%] vs. 149 [67%]; P = .0001).
- Quality of life during maintenance therapy with pemetrexed was similar to placebo, except for a small increase in loss of appetite and significantly delayed worsening of pain and hemoptysis as assessed using the Lung Cancer Symptom Scale.[48] The quality-of-life results require cautious evaluation because there was a high degree of censoring (>50%) with the primary quality-of-life end point, which was time to worsening of symptoms.
- Trials have not evaluated maintenance pemetrexed versus pemetrexed at progression.
- In the second trial, 539 patients with nonsquamous NSCLC with nonprogression after treatment with pemetrexed and cisplatin were randomly assigned to continued pemetrexed or placebo.[44]
EGFR tyrosine kinase inhibitors (TKIs) with or without chemotherapy (for patients withEGFRmutations)
Select patients with activating mutations in EGFR may benefit from single-agent EGFR TKIs. Randomized controlled trials of patients with chemotherapy-naïve NSCLC and EGFR mutations have shown that EGFR inhibitors alone improved both PFS and OS and have favorable toxicity profiles compared with combination chemotherapy. The combination of EGFR TKIs with chemotherapy showed improved PFS compared with EGFR TKI monotherapy and represents another treatment option.
Osimertinib alone
Evidence (osimertinib alone):
- A phase III, multicenter, randomized, double-blind, controlled trial (FLAURA [NCT02296125]) compared osimertinib, an oral, third-generation, irreversible EGFR TKI that inhibits both EGFR-TKI–sensitizing mutations and the EGFR T790M resistance mutation, with standard of care EGFR TKIs (gefitinib or erlotinib) as first-line treatment of patients with previously untreated, EGFR mutation-positive (exon 19 deletion or L858R), advanced NSCLC, as detected by a U.S. Food and Drug Administration (FDA)-approved test.[49] The 556 patients were randomly assigned in a 1:1 ratio.
- The primary end point of PFS was significantly longer with osimertinib (18.9 months vs. 10.2 months; HR, 0.46; 95% CI, 0.37–0.57, P < .001).[49][Level of evidence B1]
- The objective response rate was similar for both groups (80% for the osimertinib group vs. 76% for the standard EGFR TKI group).
- Central nervous system (CNS) progression was observed less often in the osimertinib group compared with the standard EGFR TKI group (6% vs. 15%).
- The median duration of response (DOR) was 17.2 months (95% CI, 13.8–22.0) with osimertinib versus 8.5 months (95% CI, 7.3–9.8) with standard EGFR TKIs.
- OS was a key secondary end point. With a follow-up of at least 39 months in each group, the median OS was 38.6 months (95% CI, 34.5–41.8) in the osimertinib group and 31.8 months (95% CI, 26.6–36.0) in the standard EGFR TKI group (HRdeath 0.80; 95.05% CI, 0.64–1.00; P = .046).[50][Level of evidence A1]
- The crossover rate from the standard EGFR TKI group to the osimertinib group was 31% (85 of 277) among patients assigned to the standard EGFR TKI group and 47% (85 of 180) among patients discontinuing the EGFR TKI. The authors noted that this crossover probably contributed to the duration of OS in the EGFR TKI (31.8 months).
- No new safety signals were observed. Rates of adverse events of grade 3 or higher and adverse events leading treatment discontinuations were similar between groups. Adverse events leading to dose interruptions, reductions, or permanent discontinuations were 43%, 5%, and 15%, respectively, in the osimertinib group and 41%, 4%, and 18%, respectively, in the EGFR TKI group.
The FDA approved osimertinib for first-line treatment of EGFR-mutant NSCLC (exon 19 deletion or L858R).
Longer PFS and OS, activity against the EGFR T790M mutation in addition to EGFR-TKI−sensitizing mutations, decreased frequency of CNS progression, and good tolerability make osimertinib the preferred choice for treatment of patients with advanced EGFR-mutated NSCLC compared with first- and second-generation EGFR TKIs.
Osimertinib plus chemotherapy
Evidence (osimertinib plus chemotherapy):
- A multicenter, randomized, open-label, phase III trial (FLAURA2 [NCT04035486]) compared osimertinib plus chemotherapy with osimertinib alone in patients with EGFR-mutated (exon 19 deletion or L858R mutation) advanced NSCLC who had not previously received treatment for advanced disease. Patients received osimertinib (80 mg once daily) with chemotherapy (pemetrexed [500 mg/m2 of body-surface area] plus either cisplatin [75 mg/m2] or carboplatin [pharmacologically guided dose, area under the curve (AUC) = 5]) or osimertinib monotherapy (80 mg once daily). Chemotherapy in the combination arm was given for four 21-day cycles and was followed by osimertinib and pemetrexed (500 mg/m2) maintenance every 3 weeks. A total of 557 eligible patients were randomly assigned in a 1:1 ratio. The primary end point was investigator-assessed PFS.[51]
- PFS was 25.5 months (24.7–not calculable) for patients in the osimertinib-plus-chemotherapy group and 16.7 months (14.1–21.3) for patients in the osimertinib-monotherapy group (HR, 0.62; 95% CI, 0.49–0.79; P < .001).[51][Level of evidence B1] PFS was assessed according to blinded independent central review and was consistent with the primary analysis (HR, 0.62; 95% CI, 0.48–0.80).
- At 24 months, 57% (95% CI, 50%–63%) of the patients in the osimertinib-plus-chemotherapy group and 41% (95% CI, 35%–47%) of those in the osimertinib-alone group were alive and progression-free.
- An objective response (complete or partial) occurred in 83% of patients who received osimertinib plus chemotherapy and 76% of patients who received osimertinib alone.
- The median DOR was 24.0 months (95% CI, 20.9–27.8) in the osimertinib-plus-chemotherapy group and 15.3 months (95% CI, 12.7–19.4), in the osimertinib-alone group.
- Grade 3 or higher adverse events from any cause were more common with the combination (64%) than with monotherapy (27%); this is consistent with known chemotherapy-related adverse events. Osimertinib plus pemetrexed with a platinum-based agent had a safety profile that was consistent with the established profiles of these agents.
Analysis of OS, a secondary end point, requires further follow-up (data maturity, 27%).
Dacomitinib
Evidence (dacomitinib):
- A multicenter, randomized, open-label, phase III trial (ARCHER 1050 [NCT01774721]) compared dacomitinib, a second-generation, irreversible EGFR TKI, administered orally at a dose of 45 mg per day with gefitinib administered orally at a dose of 250 mg per day, as first-line therapy in patients with newly diagnosed advanced NSCLC harboring the following EGFR mutations: exon 19 deletion or exon 21 L858R substitution mutations, as detected by an FDA-approved test.[52] Four hundred and fifty-two eligible patients were randomly assigned in a 1:1 ratio. The primary end point was PFS assessed by masked independent review in the intention-to-treat (ITT) population.
- Median PFS was 14.7 months in the dacomitinib group and 9.2 months in the gefitinib group (HR, 0.59; 95% CI, 0.47−0.74; P < .0001).[52][Level of evidence B1]
- The objective response rate was similar between the two groups (75% for the dacomitinib group vs. 72% for the gefitinib group; P = 0.42).
- The median DOR was longer in the dacomitinib group (14.8 months vs. 8.3 months; HR, 0.4; 95% CI, 0.31−0.53; P < .0001).
- The median OS was 34.1 months with dacomitinib vs. 26.8 months with gefitinib (HR, 0.76; 95% CI, 0.58−0.99; P = .44).[52]
- Grade 3 or higher adverse events of any cause occurred in 63% of patients who received dacomitinib and 41% of patients who received gefitinib. The most common grade 3 or 4 adverse events were dermatitis acneiform (14% in the dacomitinib group vs. none in the gefitinib group), diarrhea (8% vs. 1%), and raised alanine aminotransferase (ALT) levels (1% vs. 8%). Serious treatment-related adverse events were more frequent in the dacomitinib group (9% vs. 4%). Permanent discontinuation of the study drug because of treatment-related adverse events occurred more often in the dacomitinib group (10% vs. 7%). Dose reductions were also more frequent in the dacomitinib group (66% vs. 8%).
The FDA approved dacomitinib for first-line treatment of patients with metastatic NSCLC with EGFR exon 19 deletion or exon 21 L858R substitution mutations as detected by an FDA-approved test.
Gefitinib
Evidence (gefitinib):
- A phase III, multicenter, randomized trial compared gefitinib with carboplatin plus paclitaxel as first-line treatment in clinically selected patients in East Asia who had advanced adenocarcinoma of the lung and had never smoked or were former light smokers.[53]
- The study met its primary objective of demonstrating the superiority of gefitinib compared with the carboplatin-paclitaxel combination for PFS (HRprogression or death, 0.74; 95% CI, 0.65–0.85; P < .001).
- The median PFS was 5.7 months in the gefitinib group and 5.8 months in the carboplatin-paclitaxel group.[53][Level of evidence B1]
- Following the time that chemotherapy was discontinued and while gefitinib was continued, the PFS curves clearly separated and favored gefitinib.
- The 12-month PFS rates were 24.9% with the gefitinib group and 6.7% with the carboplatin-paclitaxel group.
- More than 90% of the patients in the trial with mutations had either del19 or exon 21 L858R mutations, which have been shown to be sensitive to EGFR inhibitors. In the subgroup of patients with a mutation, PFS was significantly longer among those who received gefitinib (HR, 0.48; 95% CI, 0.36–0.64; P < .001); however, in the subgroup of patients who were negative for a mutation, PFS was significantly longer in those who received the carboplatin-paclitaxel combination (HR with gefitinib, 2.85; 95% CI, 2.05–3.98; P < .001). There was a significant interaction between treatment and EGFR mutation with respect to PFS (P < .001).[53]
- OS was similar for patients who received gefitinib and carboplatin-paclitaxel, with no significant difference between treatments overall (HR, 0.90; 95% CI, 0.79–1.02; P = .109) or in EGFR mutation–positive (HR, 1.00; 95% CI, 0.76–1.33; P = .990) or EGFR mutation–negative (HR, 1.18; 95% CI, 0.86–1.63; P = .309; treatment by EGFR mutation interaction P = .480) subgroups. A high proportion (64.3%) of EGFR mutation–positive patients randomly assigned to the carboplatin-paclitaxel regimen received subsequent EGFR TKIs. PFS was significantly longer with gefitinib for patients whose tumors had both high EGFR gene copy number and EGFR mutation (HR, 0.48; 95% CI, 0.34–0.67) but significantly shorter when high EGFR gene copy number was not accompanied by EGFR mutation (HR, 3.85; 95% CI, 2.09–7.09).
- A phase III trial from Japan prospectively confirmed that patients with NSCLC and EGFR mutations have improved PFS but not OS when treated with gefitinib. The trial included 230 chemotherapy-naïve patients with metastatic NSCLC and EGFR mutations who were randomly assigned to receive gefitinib or carboplatin-paclitaxel.[54]
- In the planned interim analysis of data for the first 200 patients, PFS was significantly longer in the gefitinib group than in the standard-chemotherapy group (HRdeath or disease progression with gefitinib, 0.36; P < .001), resulting in early termination of the study.
- The gefitinib group had a significantly longer median PFS (10.8 months vs. 5.4 months in the chemotherapy group; HR, 0.30; 95% CI, 0.22–0.41; P < .001).[54][Level of evidence B1] The median OS was 30.5 months in the gefitinib group and 23.6 months in the standard chemotherapy group (P = .31).
- Another phase III trial from Japan also prospectively confirmed that patients with NSCLC and EGFR mutations have improved PFS but not OS when treated with gefitinib. In the second trial, the West Japanese Oncology Group conducted a phase III study (WJTOG3405) in 177 chemotherapy-naïve patients aged 75 years or younger and diagnosed with stage IIIB/IV NSCLC or postoperative recurrence harboring EGFR mutations (either the exon 19 deletion or L858R-point mutation).[55]
- Patients were randomly assigned to receive either gefitinib or cisplatin plus docetaxel (administered every 21 days for three to six cycles). The primary end point was PFS.
- The gefitinib group had significantly longer PFS than the cisplatin-plus-docetaxel group, with a median PFS of 9.2 months (95% CI, 8.0–13.9) versus 6.3 months (range, 5.8–7.8 months; HR, 0.489; 95% CI, 0.336–0.710, log-rank P < .0001).[55][Level of evidence B1]
Erlotinib
Evidence (erlotinib):
- In an open-label, randomized, phase III trial (NCT00874419) from China, 165 patients older than 18 years with histologically confirmed stage IIIB/IV NSCLC and a confirmed activating mutation of EGFR (i.e., exon 19 deletion or exon 21 L858R-point mutation) received either oral erlotinib (150 mg/day) until they experienced disease progression or unacceptable toxic effects, or up to four cycles of gemcitabine plus carboplatin.[56]
- Median PFS was significantly longer in erlotinib-treated patients than in patients treated with chemotherapy (13.1 months [95% CI, 10.58–16.53] vs. 4.6 months [range, 4.21–5.42 months]; HR, 0.16; 95% CI, 0.10–0.26; P < .0001).[56][Level of evidence B1]
- In a European study (EURTAC [NCT00446225]), 1,227 patients with advanced NSCLC were screened for EGFR mutations. Of these, 174 patients with EGFR mutations were randomly assigned to receive erlotinib or platinum-based chemotherapy.[57] The primary end point was PFS.
- In an interim analysis of the first 153 patients, PFS in the chemotherapy arm was 5.2 months (95% CI, 4.5–5.8) compared with 9.7 months (95% CI, 8.4–12.3) in the erlotinib arm (HR, 0.37; P < .0001). Median survival was 19.3 months in patients in the chemotherapy arm and 19.5 months in patients in the erlotinib arm (HR, 0.80; P = .42).[58][Level of evidence B1]
Afatinib
Evidence (afatinib):
- In an open-label, randomized, phase III study (LUX-Lung 3 [NCT00949650]), 345 Asian (72%) and White (26%) patients with stage IIIB/IV NSCLC and confirmed EGFR mutations (i.e., exon 19 deletion, L858R, or other [38 of 345 patients had other less-common mutations]) were screened, and 340 patients received at least one dose of study medication, which was either 40 mg of oral afatinib, an irreversible EGFR/human epidermal receptor TKI, daily or up to six cycles of cisplatin and pemetrexed for first-line treatment.[59]
- The primary end point was PFS. In this study, the afatinib group had significantly longer PFS than the cisplatin-plus-pemetrexed group, with a median PFS of 11.1 months for afatinib and 6.9 months for chemotherapy (HR, 0.58; 95% CI, 0.43–0.78; P = .001).[59][Level of evidence B1]
- Assessment of OS was a secondary end point and was reported separately.[60] Similar to the PFS analysis, OS was stratified on the basis of EGFR-mutation type and ethnic origin.
- With a median follow-up of 41 months, median OS was 28.2 months in patients in both arms (HR, 0.88; 95% CI, 0.66–1.17; P = .39).
- In patients harboring common EGFR mutations (i.e., exon 19 deletion and L858R), survival did not differ significantly between treatment arms (HR, 0.78; 95% CI, 0.58–1.06; P = .11). However, prespecified subgroup analyses demonstrated a survival advantage with afatinib compared with chemotherapy in patients with tumors harboring the EGFR del19 mutation (median OS, 33.3 months vs. 21.1 months; HR, 0.54; 95% CI, 0.36–0.79; P = .0015) but no significant difference between treatment arms in patients with tumors harboring the L858R mutation (median OS, 27.6 months vs. 40.3 months; HR, 1.30; 95% CI, 0.80–2.11; P = .29).
- First-line afatinib was associated with a significant survival advantage compared with chemotherapy in patients with NSCLC-harboring EGFR del19 mutations but not in patients with EGFR L858R mutations or in the overall EGFR–mutation-positive patient population.[60][Level of evidence A1]
- In an open-label, randomized, phase III study (LUX-Lung 6 [NCT01121393]), 364 East Asian patients with stage IIIB/IV NSCLC and confirmed EGFR mutations (i.e., exon 19 deletion, L858R, or other) were randomly assigned (in a 2:1 ratio) to 40 mg of afatinib daily or gemcitabine and cisplatin for up to six cycles for first-line treatment.[61]
- The primary end point was PFS. Median PFS was significantly longer in the afatinib group (11.0 months; 95% CI, 9.7–13.7) than in the gemcitabine and cisplatin group (5.6 months, [range, 5.1–6.7 months]; HR, 0.28; 95% CI, 0.20–0.39; P < .0001).[61][Level of evidence B1]
- Assessment of OS was a prespecified secondary end point and was reported separately.[60] Similar to the PFS analysis, OS was stratified on the basis of EGFR-mutation type and ethnic origin.
- With a median follow-up of 33 months, median OS was 23.1 months in patients in the afatinib arm and 23.5 months in patients in the chemotherapy arm (HR, 0.93; 95% CI, 0.72–1.22; P = .61).
- In patients harboring common EGFR mutations (i.e., exon 19 deletion and L858R), survival did not differ significantly between treatment arms (HR, 0.83; 95% CI, 0.62–1.09; P = .18). However, prespecified subgroup analyses demonstrated a survival advantage with afatinib compared with chemotherapy in patients with tumors harboring the EGFR del19 mutation (median OS, 31.4 months vs. 18.4 months; HR, 0.64; 95% CI, 0.44–0.94; P = .023), but no significant difference between treatment arms was seen in patients with tumors harboring the L858R mutation (median OS, 19.6 months vs. 24.3 months; HR, 1.22; 95% CI, 0.81–1.83; P = .34).
- First-line afatinib was associated with a significant survival advantage compared with chemotherapy in patients with NSCLC-harboring EGFR del19 mutations but not in patients with EGFR L858R mutations or in the overall EGFR-mutation-positive patient population.[60][Level of evidence A1]
EGFR-directed therapy (for patients withEGFRexon 20 insertion mutations)
Amivantamab
Amivantamab has been previously approved for patients with locally advanced or metastatic NSCLC harboring EGFR exon 20 insertion mutations whose disease has progressed on or after platinum-based chemotherapy.
Evidence (amivantamab plus chemotherapy):
- PAPILLON (NCT04538664) was a phase III randomized trial that compared amivantamab plus chemotherapy with chemotherapy alone as first-line treatment for patients with advanced NSCLC and EGFR exon 20 insertions. Patients in the chemotherapy-alone group who had disease progression were allowed to cross over to receive amivantamab monotherapy. A total of 308 patients were randomly assigned 1:1 to receive either amivantamab plus carboplatin and pemetrexed or carboplatin plus pemetrexed. The primary end point was PFS according to blinded independent central review. The median follow-up was 14.9 months.[62][Level of evidence B1]
- At 18 months, the PFS rate was 31% for patients who received amivantamab plus chemotherapy, and 3% for patients who received chemotherapy alone.
- The objective response rate was higher in the amivantamab-plus-chemotherapy group (73%) than the chemotherapy-alone group (47%).
- In an interim OS analysis, there was no statistically significant difference.
- The most common adverse events with amivantamab plus chemotherapy were hematologic toxicities, rash, and paronychia. Infusion reactions occurred in 42% of patients.
The study supports amivantamab plus chemotherapy as an effective first-line treatment option for patients with NSCLC and EGFR exon 20 insertions based on superior PFS when compared with chemotherapy alone.[62]
ALK inhibitors (for patients withALKtranslocations)
Alectinib
Evidence (alectinib):
- In an open-label, randomized, phase III study (the ALEX trial [NCT02075840]), 303 patients with previously untreated, advanced ALK-rearranged NSCLC received either alectinib (600 mg twice a day) or crizotinib (250 mg twice a day).[63] The primary end point was investigator-assessed PFS.
- The rate of PFS was significantly higher with alectinib than crizotinib; the 12-month event-free survival rate was 68.4% for the alectinib group (95% CI, 61.0%–75.9%) compared with 48.7% for the crizotinib group (95% CI, 40.4%–56.9%) (HR, 0.47; 95% CI, 0.34–0.65; P < .001). The median PFS was not reached with alectinib. The results of independent review committee-assessed PFS were consistent.[63][Level of evidence B1]
- CNS progression events were less frequent with alectinib (12%) than with crizotinib (45%) (HR, 0.16; 95% CI, 0.10–0.28; P <.001).
- The response rate was similar for both groups, 82.9% for the alectinib group compared with 75.5% for the crizotinib group (P = .09).
- Grade 3 to 5 adverse events were less frequent with alectinib (41%) than with crizotinib (50%).
- A second, open-label, randomized, phase III trial (J-ALEX) recruited 207 ALK-inhibitor–naïve Japanese patients with ALK-positive NSCLC who were chemotherapy-naïve or had received one previous chemotherapy regimen. Patients were randomly assigned in a 1:1 ratio to receive alectinib (300 mg twice daily, which is the dose approved in Japan and is lower than the 600 mg twice daily dose approved elsewhere) versus crizotinib (250 mg twice daily).[64] The primary end point was PFS-assessed by an independent review committee.
- At data cutoff for the second primary interim analysis, the independent data monitoring committee determined that the primary end point was met (HR, 0.34; 99.7% CI, 0.17–0.71; P <.0001) and recommended immediate release of the data. Median PFS had not been reached with alectinib but was reached at 10.2 months with crizotinib.
- Grade 3 or 4 adverse events occurred less frequently with alectinib (26% occurrence rate) than with crizotinib (52% occurrence rate).
Lorlatinib
Evidence (lorlatinib):
- The phase III CROWN trial (NCT03052608) included patients with advanced ALK-rearranged NSCLC who had received no prior systemic therapy for metastatic disease. The trial randomly assigned 296 patients to receive either lorlatinib (100 mg daily) or crizotinib (250 mg twice daily). The primary end point was PFS as determined by blinded independent central review.[65][Level of evidence B1]
- A planned interim analysis was conducted after approximately 75% of expected events of disease progression or death had occurred. The percentage of patients alive without progression at 12 months was 78% (95% CI, 70%–84%) in the lorlatinib group and 39% (95% CI, 30%–48%) in the crizotinib group. Objective responses occurred in 76% of patients who received lorlatinib and 58% of patients who received crizotinib.
- Post hoc analysis showed improved PFS for the lorlatinib group, compared with the crizotinib group in patients with and without brain metastases at baseline (12-month PFS rates: 78% vs. 22% and 78% vs. 45%, respectively).[66]
- There was a lower 12-month cumulative incidence of CNS progression with lorlatinib, compared with crizotinib in patients with (7% vs. 72%) and without (1% vs. 18%) brain metastases at baseline.
- Lorlatinib was associated with more grade 3 to 4 adverse events than crizotinib (72% vs. 56%), the most common being altered lipid levels. Treatment discontinuation occurred in 7% of patients who received lorlatinib and 9% of patients who received crizotinib.[65]
- Updated safety data in the post hoc analysis showed that 35% of patients had CNS adverse events with lorlatinib, most of grade 1 severity. These included memory impairment and mood effects, including anxiety, depression, and lability. The occurrence of CNS adverse events did not result in a clinically meaningful difference in patient-reported quality of life. At analysis, 56% of CNS adverse effects had resolved (33% without intervention; 17% with lorlatinib dose modification), and 38% were unresolved. Lorlatinib dose modification did not notably influence PFS.
The FDA approved lorlatinib for patients with metastatic NSCLC whose tumors are ALK-positive, as detected by an FDA-approved test.
Crizotinib
Evidence (crizotinib):
- In an open-label, randomized, phase III study, 343 patients with stage IIIB/IV NSCLC harboring translocations in ALK received either 250 mg of crizotinib orally twice a day or the combination of pemetrexed and cisplatin or carboplatin for up to six cycles.[67] At the time of disease progression, patients on the chemotherapy arm were allowed to cross over to crizotinib; 60% of patients in the chemotherapy arm subsequently received crizotinib. The primary end point of this study was PFS.
- The study met its primary end point and demonstrated that crizotinib is superior to chemotherapy in prolonging PFS (median, 10.9 months vs. 7.0 months; HR, 0.454; 95% CI, 0.346–0.596; P < .0001).[68][Level of evidence B1]
Ceritinib
Evidence (ceritinib):
- In an open-label, randomized, phase III study, 376 patients with stage IIIB/IV ALK-rearranged nonsquamous NSCLC received either oral ceritinib 750 mg daily or platinum-based chemotherapy (cisplatin or carboplatin and pemetrexed) every 3 weeks for four cycles, followed by maintenance pemetrexed.[69] The primary end point was PFS and crossover from chemotherapy to ceritinib was allowed upon documented progression.
- Median PFS, assessed by blinded independent review, was 16.6 months in the ceritinib group and 8.1 months in the chemotherapy group (HR, 0.55; 95% CI, 0.42–0.73; P < .00001).
- The median OS was not reached with ceritinib, and it was 26.2 months with chemotherapy (HR, 0.73; 95% CI, 0.50–1.08; P = .056).[69][Level of evidence B1]
Brigatinib
Evidence (brigatinib):
- A phase II, open-label trial (NCT02094573) enrolled 222 patients with ALK-translocated locally advanced or metastatic NSCLC who had disease progression after crizotinib treatment. Patients were randomly assigned to receive 90 mg every day (n = 112; 109 treated) or 180 mg every day with a 7-day lead-in at 90 mg every day (n = 110).[70]
- The primary end point assessed by the investigators was objective response rate. The objective response rate was 45% (97.5% CI, 34%–56%) for patients who received the 90 mg dose and 54% (97.5% CI, 43%–65%) for patients who received the 180 mg dose.
- Median PFS was 9.2 months (95% CI, 7.4–15.6) for patients who received the 90 mg dose and 12.9 months (95% CI, 11.1–not reached) for patients who received the 180 mg dose.
- At data cutoff, the median DOR was 13.8 months (95% CI, 5.6–13.8) for patients who received the 90 mg dose and 11.1 months (95% CI, 9.2–13.8) for patients who received the 180 mg dose.[70][Level of evidence B3]
- The CNS objective response rate in patients with measurable CNS lesions was 42% in patients who received 90 mg every day (n = 26) and 67% in patients who received 180 mg every day (n = 18).
- Common adverse events, which were mainly grade 1 or 2 and occurred in 27% to 38% of patients at the higher dose, were nausea, diarrhea, headache, and cough. A subset of pulmonary adverse events with early onset (median onset, day 2) occurred in 14 of 219 treated patients (all grades, 6%; grade ≥3, 3%); none occurred after escalation to 180 mg. These events included dyspnea, hypoxia, cough, pneumonia, or pneumonitis. They were managed with dose interruption. Seven of the 14 patients were successfully retreated with brigatinib.
- The FDA-approved dose of brigatinib is 90 mg every day for 7 days; if tolerated, the dose is increased to 180 mg every day.
BRAF V600E and MEK inhibitors (for patients withBRAFV600E mutations)
BRAF V600E mutations occur in 1% to 2% of lung adenocarcinomas.
Dabrafenib and trametinib
Evidence (dabrafenib and trametinib):
- In a phase II, multicenter, nonrandomized, open-label study (NCT01336634), 36 patients with previously untreated metastatic NSCLC who tested positive for BRAF V600E mutations were treated with dabrafenib (a BRAF inhibitor) 150 mg twice a day and trametinib (a MEK inhibitor) 2 mg every day.[71]BRAF V600E mutations were identified by the Oncomine Dx Target Test (ThermoFisher Scientific). The primary end point was investigator-assessed overall response.
- The overall response rate was 64% (95% CI, 46%–79%). Six percent of patients had a complete response, and 58% of patients had a partial response.
- The median investigator-assessed PFS was 10.9 months (95% CI, 7.0–16.6). The estimated median DOR was 10.4 months (95% CI, 8.3–17.9). At data cutoff, 47% of patients had died, and the median OS was 24.6 months (95% CI, 12.3–not estimable).
- Sixty-nine percent of patients had at least one grade 3 or 4 adverse event, of which the most common were pyrexia, ALT increase, hypertension, or vomiting. Adverse events led to permanent discontinuation in 22% of patients, dose interruption or delay in 75% of patients, and dose reduction in 39% of patients.[71][Level of evidence C3]
The FDA approved the combination of dabrafenib and trametinib in the treatment of patients with NSCLC whose tumors harbor BRAF V600E mutations as detected by an FDA-approved test.
ROS1 inhibitors (for patients withROS1rearrangements)
ROS1 rearrangements occur in approximately 1% of patients with NSCLC.[72] The FDA approved crizotinib and entrectinib for use in patients with NSCLC and ROS1 rearrangements, with the latter appearing to have greater activity against intracranial disease.
Entrectinib
The FDA approved entrectinib for treatment of patients with metastatic NSCLC whose tumors are ROS1-positive, regardless of the number of previous systemic therapies.
Evidence (entrectinib):
- The safety and clinical activity of entrectinib in ROS1 fusion-positive metastatic NSCLC was determined by integrated analysis of three multicenter, single-arm, open-label clinical trials (ALKA-372-001/EudraCT, 2012-000148-88, STARTRK-1 [NCT02097810], and STARTRK-2 [NCT02568267]).[73] Entrectinib was administered orally at a dose of at least 600 mg once daily. Primary end points were the objective response rate and the DOR determined by blinded independent central review. Of note, time-to-event end points are difficult to interpret in the absence of a control arm. Evaluation of tumor samples for the ROS1 gene fusion was conducted prospectively in local laboratories using either a FISH or next-generation sequencing (NGS) laboratory-developed test.
Seventeen (32%) patients had received no previous systemic therapy, 23 (43%) had received one previous therapy, and 13 (25%) had received two or more lines of treatment. CNS disease was present in 23 (43%) patients at baseline. Thirty-one (59%) patients were never-smokers and 52 (98%) patients had adenocarcinoma histology.
- The objective response rate in 53 efficacy-evaluable patients was 77% (95% CI, 64%−88%). Six percent of patients had a complete response and 72% had a partial response. Among patients with CNS disease at baseline, the objective response rate was 74% (95% CI, 52%−90%) and all patients had a partial response. Among patients without CNS disease at baseline, the overall response rate was 80% (95% CI, 61%−92%) (10% had a complete response and 70% had a partial response).[73][Level of evidence C3]
- The median DOR was 24.6 months (95% CI, 11.4−34.8) in efficacy-evaluable patients; 12.6 months (95% CI, 6.5−not estimable) in patients with baseline CNS disease, and 24.6 months (95% CI, 11.4−34.8) in those without CNS disease at baseline.
- Treatment-related adverse events were assessed in 134 patients in the safety-evaluable population. Grade 1 or 2 treatment-related adverse events were observed in 79 patients (59%). Grade 3 or 4 treatment-related adverse events were observed in 46 patients (34%). Fifteen patients (11%) had serious treatment-related adverse events. There were no treatment-related deaths.
- The median PFS was 19 months (95% CI, 12.2−36.6) in efficacy-evaluable patients; 13.6 months (95% CI, 4.5−not estimable) in patients with baseline CNS disease, and 26.3 months (95% CI, 15.7−36.6) in patients with no baseline CNS disease.
Crizotinib
Crizotinib was approved for patients with metastatic NSCLC whose tumors are ROS1-positive, regardless of the number of previous systemic therapies.
Evidence (crizotinib):
- In an expansion cohort of a phase I study of crizotinib, 50 patients with advanced NSCLC who tested positive for ROS1 rearrangement were treated with oral crizotinib 250 mg twice daily.[74]ROS1 rearrangements were identified using break-apart FISH or reverse transcriptase−polymerase chain reaction assay. Seven patients (14%) had not had any previous treatment for advanced disease, 21 patients (42%) had one prior treatment, and 22 patients (44%) had more than one prior treatment. The primary end point was response rate.
- The overall response rate was 72% (95% CI, 58%–84%). Six percent of patients had a complete response, 66% had a partial response, and 18% had stable disease as their best response.
- Median PFS was 19.2 months (95% CI, 14.4–not reached). The estimated DOR was 17.6 months (95% CI, 14.5–not reached).[74][Level of evidence C3]
- In a phase II, open-label, single-arm trial, 127 East Asian patients with ROS1-positive NSCLC were treated with crizotinib 250 mg twice daily.[75] Twenty-four patients (18.9%) had not had any previous treatment for advanced disease, 53 patients (41.7%) had one previous treatment, and 50 patients (39%) had two or three previous treatments. The primary end point was objective response rate by independent review.
- The objective response rate was 71.7% (95% CI, 63.0%–79.3%). Response rates were similar, irrespective of the number of previous therapies. Complete responses occurred in 13.4% of patients, while 58.3% of patients had partial responses, and 16.5% of patients had stable disease as their best response.[75][Level of evidence C3]
- Median PFS was 15.9 months (95% CI, 12.9–24). The DOR was 19.7 months (95% CI, 14.1–not reached).
- OS was 32.5 months (95% CI, 32.5–not reached).
NTRK inhibitors (for patients withNTRKfusions)
Somatic gene fusions in NTRK occur across a range of solid tumors including in fewer than 0.5% of NSCLC tumors.[76,77] These fusions appear to occur more frequently in nonsmokers with lung adenocarcinoma.
Larotrectinib
Evidence (larotrectinib):
- Larotrectinib was studied in three protocols: a phase I study involving adults, a phase I/II study involving children, and a phase II study involving adolescents and adults.[78] Fusions were confirmed in the tumors using either FISH or NGS methods. The primary end point for the combined analysis was objective response rate by independent review and was conducted with input from regulators with the goal of excluding a lower bound of less than 30% for response rate. In total, 55 patients with a median age of 45 years (range, 4 months‒76 years) were enrolled across 17 different NTRK fusion-positive tumor types. All patients had either metastatic disease (82%) or locally advanced unresectable disease (18%). Enrolled patients had received a median of two previous systemic therapies.
- The objective response rate was 75% (95% CI, 61%‒75%) and 73% of these responses lasted at least 6 months.[78][Level of evidence C3]
- Treatment was well tolerated with 93% of adverse events being grade 1 to 2; the most common grade 3 to 4 adverse events were anemia (11% of patients), transaminitis (7%), and neutropenia (7%).
The FDA approved larotrectinib for the treatment of patients who have locally advanced or metastatic tumors that harbor an NTRK gene fusion without a known acquired resistance mutation, and who have no satisfactory alternative treatments or whose cancer has progressed following treatment.
Entrectinib
The FDA granted accelerated approval to entrectinib for the treatment of solid tumors that have an NTRK gene fusion without a known acquired resistance mutation, are metastatic, have progressed after treatment, have no satisfactory alternative therapy, or for cases in which surgical resection is likely to result in severe morbidity.
Evidence (entrectinib):
- The safety and clinical activity of entrectinib in NTRK inhibitor-naïve patients with metastatic or locally-advanced solid tumors (including NSCLC) harboring NTRK1, NTRK2, or NTRK3 gene fusions was determined by integrated analysis of three early-phase, multicenter, single-arm, open-label clinical trials (ALKA-372-001/EudraCT, 2012-000148-88, STARTRK-1 [NCT02097810], and STARTRK-2 [NCT02568267]).[79] Treatment consisted of entrectinib administered orally at a dose of at least 600 mg once per day. The primary end points were objective response rate and median DOR, which were assessed by blinded independent central review. Of note, time-to-event end points are difficult to interpret in the absence of a control arm. Identification of positive NTRK gene fusion status was conducted prospectively in local laboratories or a central laboratory using various nucleic acid–based tests.
Of 54 patients in the NTRK gene fusion-positive efficacy-evaluable population, 20 (37%) had received no previous systemic therapy, 11 (20%) had received one previous systemic therapy, and 23 (43%) had received two or more systemic therapies. Twelve (22%) patients had CNS disease at baseline. Ten (19%) patients had NSCLC. Fifty-two (96%) patients had an NTRK gene fusion detected by NGS and 2 (4%) had an NTRK gene fusion detected by other nucleic acid–based tests.
- The objective response rate in 54 patients was 57% (95% CI, 43.2%−70.8%). Seven percent of patients had a complete response and 50% had a partial response. In patients with baseline CNS disease, 50% achieved a response (all partial responses), whereas in patients without baseline CNS disease, 60% achieved a response (10% complete response; 50% partial response).[79][Level of evidence C3]
- The median DOR in efficacy-evaluable patients was 10.4 months (95% CI, 7.1−not estimable). In patients with baseline CNS disease DOR was not estimable, and in patients with no baseline CNS disease it was 12.9 months (95% CI, 7.1−not estimable).
- Among 10 patients with NSCLC, the response rate was 70% (95% CI, 35%−93%) and DOR ranged between 1.9 months and 20.1 months. For more information, see the prescribing information.
- The safety-evaluable population consisted of 68 patients with NTRK fusion-positive tumors. Most treatment-related adverse events were grade 1 or 2 and reversible. The most frequent grade 3 or 4 treatment-related adverse events were increased weight gain (10%) and anemia (12%). Serious treatment-related adverse events were reported in 7 (10%) patients. Three (4%) patients had dose interruptions and 27 (40%) patients had dose reductions due to treatment-related adverse events. There were no treatment-related deaths.
- Median PFS was 11.2 months (95% CI, 8.0−14.9). In patients with baseline CNS disease, median PFS was 7.7 months (95% CI, 4.7−not estimable), and it was 12 months (95% CI, 8.7−15.7) in patients with no baseline CNS disease.
RET inhibitors (for patients withRETfusions)
Somatic gene fusions of RET occur in 1% to 2% of patients with NSCLC and in patients with thyroid cancer.[80]
Selpercatinib
Evidence (selpercatinib):
- A phase I/II study (LIBRETTO-001 [NCT03157128]) enrolled patients with RET fusion−positive solid tumors. RET fusion status was determined by local molecular testing (NGS, FISH, or polymerase chain reaction assay) without central confirmation. The primary end point was objective response.[81][Level of evidence C3]
- Updated analysis was conducted in 316 patients with RET fusion–positive NSCLC.[81]
- Among the 69 treatment-naïve patients, the objective response rate was 84% (95% CI, 73%–92%), and 6% achieved complete responses. The median DOR was 20.2 months (95% CI, 13.0–could not be evaluated); 40% of responses were ongoing at the data cutoff (median follow-up, 20.3 months). The median PFS was 22.0 months; 35% of patients were alive and progression-free at the data cutoff (median follow-up, 21.9 months).
- Among the 247 patients who had received prior platinum-based chemotherapy, the objective response rate was 61% (95% CI, 55%–67%), and 7% achieved complete responses. The median DOR was 28.6 months (95% CI, 20.4–could not be evaluated); 49% of responses were ongoing (median follow-up, 21.2 months). The median PFS was 24.9 months; 38% of patients were alive and progression-free at the data cutoff (median follow-up, 24.7 months).
- Among the 26 patients with measurable baseline CNS metastasis by the independent review committee, the intracranial objective response rate was 85% (95% CI, 65%–96%), and 27% had complete responses.
- In the full safety population (n = 796), the median treatment duration was 36.1 months.
- There was no significant change in the safety profile. Most adverse events were grade 1 to 2. The most common adverse events were edema, diarrhea, fatigue, dry mouth, hypertension, increased ALT and aspartate aminotransferase (AST), and rash.
The FDA approved selpercatinib to treat adults with locally advanced or metastatic NSCLC with RET gene fusion, as detected by an FDA-approved test.
Pralsetinib
Evidence (pralsetinib):
- A phase I/II study (ARROW [NCT03037385]) enrolled patients with RET fusion−positive solid tumors. Two hundred thirty-three patients had RET fusion−positive NSCLC. RET fusion status was determined by local molecular testing of tumor or circulating tumor nucleic acid (ctDNA) in blood, without central confirmation. The primary end point was objective response.[82][Level of evidence C3]
- Ninety-two patients who had received platinum-based chemotherapy and 29 patients who were treatment-naive (and not candidates for standard platinum-based treatment) received pralsetinib before the efficacy enrollment cutoff (July 11, 2019). Eighty-seven previously treated patients and 27 treatment-naive patients had centrally adjudicated baseline measurable disease, and thus formed the efficacy cohort.
- The overall response rate was 61% (95% CI, 50%–71%) in the 87 patients who had received platinum-based chemotherapy, including complete responses in 6%. The median DOR was not reached (15.2 months–not estimable).
- The overall response rate was 70% (95% CI, 50%–86%) in the 27 treatment-naive patients, including complete responses in 11%. The median DOR was 9.0 months (6.3–not estimable).
- In the 233-patient safety cohort, 93% had treatment-related adverse events, including 48% with grade 3 or worse events. The most common grade 3 or worse treatment-related adverse events were neutropenia (18%), hypertension (11%), and anemia (10%). Dose reductions occurred in 38% of patients, and 6% discontinued treatment because of adverse events.
MET inhibitors (for patients withMETexon 14 skipping mutations)
Dysregulation of the MET proto-oncogene resulting from disruption of distinct splice sites leads to loss of MET exon 14 and enhanced MET signaling. These MET alterations drive tumor proliferation, survival, invasion, and metastasis, and occur in 3% to 4% of patients with NSCLC.[83]
Tepotinib
Evidence (tepotinib):
- An open-label phase II study (VISION [NCT02864992]) enrolled patients with MET exon 14 skipping mutations. The trial included 152 patients who received tepotinib (500 mg orally once daily). MET status was determined centrally, either via liquid biopsy (from circulating free DNA obtained from plasma; n = 66) or via tissue biopsy (n = 60). Twenty-seven patients had positive results from both methods. The primary end point was objective response.[84][Level of evidence C3]
- Among the 99 patients who had been followed for at least 9 months (i.e., the efficacy population), the objective response rate as assessed by independent review was 46% (95% CI, 36%–57%), with a median DOR of 11.1 months (95% CI, 7.2–not estimable). Response rates were similar in the liquid biopsy and tissue biopsy groups.
- Responses were similar regardless of prior therapy.
- Grade 3 or higher adverse events occurred in 28% of patients, including peripheral edema in 7% of patients. Adverse events led to therapy discontinuation in 11% of patients.
Capmatinib
Evidence (capmatinib):
- A phase II study (GEOMETRY [NCT02414139]) evaluated capmatinib (400 mg orally twice daily) in patients with MET exon 14 skipping mutations or MET amplification. MET status was performed centrally. A total of 364 patients were enrolled. The primary end point was overall response.[85][Level of evidence C3]
- Of the 69 patients with MET exon 14 skipping mutations who had received one or two prior lines of therapy, the overall response rate was 41% (95% CI, 29%–53%). The median DOR was 9.7 months (95% CI, 5.6–13.0).
- Of the 28 patients with MET exon 14 skipping mutations who had not received any prior treatment, the overall response rate was 68% (95% CI, 48%–84%). The median DOR was 12.6 months (95% CI, 5.6–not estimable).
- Response rates in patients with MET amplification without the exon 14 skipping mutation did not meet the prespecified threshold for clinically relevant activity.
- Grade 3 to 4 adverse events of occurred in 67% of patients. The most common events, regardless of causality, were peripheral edema, nausea, vomiting, and increased creatinine. Adverse events led to therapy discontinuation 11% of patients.
Immune checkpoint inhibitors with or without chemotherapy
Pembrolizumab is a humanized monoclonal antibody that inhibits the interaction between the programmed death protein 1 (PD-1) coinhibitory immune checkpoint expressed on tumor cells and infiltrating immune cells and its ligands, PD-L1 and PD-L2.[86]
Pembrolizumab plus chemotherapy
Evidence (pembrolizumab plus chemotherapy):
- A phase III double-blind trial (KEYNOTE-189 [NCT02578680]) randomly assigned, in a 2:1 ratio, 616 patients with metastatic nonsquamous NSCLC without sensitizing EGFR mutations or ALK rearrangements who had received no previous treatment for metastatic disease. Patients received pemetrexed and a platinum-based drug plus either 200 mg of pembrolizumab or placebo every 3 weeks for 4 cycles, followed by pembrolizumab or placebo for up to a total of 35 cycles plus pemetrexed maintenance.[1] Crossover to pembrolizumab monotherapy was permitted after verified progression among patients in the placebo-containing combination group. The primary end points were OS and PFS as assessed by blinded independent central committee radiological review.
- In the 5-year updated analysis, the median time from random assignment to data cutoff was 64.6 months (range, 60.1–72.4).[87]
- After 5 years, pembrolizumab plus pemetrexed-platinum was associated with improved OS and PFS, compared with placebo plus pemetrexed-platinum in patients with metastatic nonsquamous NSCLC, regardless of PD-L1 expression. In the ITT population, 5-year OS rates were 19.4% in the pembrolizumab plus pemetrexed-platinum group, compared with 11.3% in the placebo plus pemetrexed-platinum group.
- Survival was higher in patients with a higher PD-L1 tumor proportion score (TPS), especially in the TPS >50% subgroup (29.6% vs. 21.4%).
- There were 57 patients who completed 35 cycles of pembrolizumab. For these patients, the objective response rate was 86.0% and the 3-year OS rate after completing 35 cycles (approximately 5 years after random assignment) was 71.9%.[87]
- Immune-mediated adverse events and infusion reactions occurred in 113 (27.9%) and 27 (13.4%) patients.
- Adverse events of grade 3 or higher occurred with similar frequency in both treatment groups (71.9% in the pembrolizumab combination group vs. 66.8% in the placebo combination group).
- A phase III, randomized, double-blind study (KEYNOTE-407 [NCT02775435]) included previously untreated patients with metastatic squamous cell NSCLC. Patients were randomly assigned 1:1 to receive pembrolizumab 200 mg or placebo plus carboplatin and paclitaxel/nab-paclitaxel once every 3 weeks for four cycles, followed by pembrolizumab or placebo for up to 35 cycles (pembrolizumab-plus-chemotherapy, n = 5,278; placebo-plus-chemotherapy, n = 5,281). Primary end points were OS and PFS per RECIST version 1.1 by blinded independent central review.[88]
- The median time from random assignment to data cutoff was 56.9 months (range, 49.9–66.2). OS and PFS were improved with pembrolizumab-plus-chemotherapy versus placebo-plus-chemotherapy (HR, 0.71 [0.59–0.85] and 0.62 [0.52–0.74]), respectively; 95% CI). The 5-year OS rates were 18.4% and 9.7%, respectively.[88][Level of evidence A1]
- A total of 55 patients completed 35 cycles of pembrolizumab. The objective response rate was 90.9% and the 3-year OS rate after completion of 35 cycles (approximately 5 years after random assignment) was 69.5%.
Pembrolizumab alone
Evidence (pembrolizumab alone):
- A phase III open-label study (KEYNOTE-024) randomly assigned 305 patients with previously untreated, advanced NSCLC with PD-L1 expression on 50% or more tumor cells and no sensitizing EGFR mutations or ALK translocations to either intravenous pembrolizumab (200 mg every 3 weeks for up to 35 cycles) or platinum-based chemotherapy (four to six cycles, investigator's choice; pemetrexed maintenance was allowed for nonsquamous tumors).[86] The primary end point was PFS.
- PD-L1 expression was centrally assessed using the PD-L1 immunohistochemistry 22C3 pharmDx assay. PD-L1 tumor expression of 50% or more was found in 30.2% of 1,653 patient samples that were examined.
- Pembrolizumab demonstrated significant improvement in median PFS (10.3 months vs. 6.0 months; HR, 0.50; 95% CI, 0.37–0.68; P < .001). The overall response rate (44.8% vs. 27.8%), the median DOR (not reached, [range, 1.9–14.5 months] vs. 6.3 months [range, 2.1–12.6 months]), and the estimated rate of OS at 6 months (80.2% vs. 72.4%; HR, 0.60; 95% CI, 0.41–0.89; P = .005) were all higher with pembrolizumab than with chemotherapy.
- Further follow-up of this study confirmed an OS advantage in favor of pembrolizumab; the median OS for patients who received pembrolizumab was 30 months (95% CI, 18.3 months–not reached) versus 14.2 months for patients who received chemotherapy, with a 75% crossover to immunotherapy afterwards, suggesting the crossover did not impact survival.[89]
- Adverse events (any grade) were less frequent with pembrolizumab than with chemotherapy (73.4% vs. 90.0%).
- Grade 3 to 5 adverse events occurred in 26.6% of patients treated with pembrolizumab and 53.3% of patients treated with chemotherapy.
- Grade 3 or 4 immune-related events occurred in 9.7% of patients treated with pembrolizumab and 0.7% of patients treated with chemotherapy.
- The most common grade 3 or 4 immune-related events associated with pembrolizumab were severe skin reactions (3.9%), pneumonitis (2.6%), and colitis (1.3%).
- There were no grade 5 immune-related events.
- Pembrolizumab treatment demonstrated significant improvement in PFS, OS, and DOR with less frequent adverse events compared with chemotherapy treatment.[86][Level of evidence B1]
- A phase III open-label study (KEYNOTE-042 [NCT02220894]) included patients with locally advanced or metastatic NSCLC without EGFR or ALK alterations and with a PD-L1 TPS score greater than 1%. Patients were randomly assigned to receive either pembrolizumab 200 mg once every 3 weeks for 35 cycles or chemotherapy (carboplatin plus paclitaxel or pemetrexed) for four to six cycles with optional maintenance pemetrexed (pembrolizumab, n = 637; chemotherapy, n = 637). The primary end points were OS in the populations with a PD-L1 TPS greater than 50%, greater than 20%, and greater than 1%.[90]
- The median follow-up was 61.1 months (range, 50.0–76.3).
- OS outcomes favored pembrolizumab versus chemotherapy, regardless of the PD-L1 TPS.
- The HR was 0.68 (95% CI, 0.57–0.81) for the TPS >50% group, 0.75 (95% CI, 0.64–0.87) for the TPS >20% group, and 0.79 (95% CI, 0.70–0.89) for the TPS >1% group.
- The OS rates for patients who received pembrolizumab were 21.9% (TPS >50%), 19.4% (TPS >1%), and 16.6% (TPS >1%).
- The most common adverse reactions reported in at least 10% of patients who received pembrolizumab as a single agent in KEYNOTE-042 included fatigue, decreased appetite, dyspnea, cough, rash, constipation, diarrhea, nausea, hypothyroidism, pneumonia, pyrexia, and weight loss.
The FDA approved pembrolizumab in combination with pemetrexed and carboplatin as first-line treatment of patients with metastatic nonsquamous NSCLC, regardless of PD-L1 expression. The FDA also approved pembrolizumab as a first-line monotherapy for patients with NSCLC whose tumors express PD-L1 (>1%) (staining as determined by an FDA-approved test). Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapies before receiving pembrolizumab (see the FDA label for pembrolizumab).
Cemiplimab-rwlc plus chemotherapy
Evidence (cemiplimab-rwlc plus chemotherapy):
- A phase III, double-blind, placebo-controlled trial (EMPOWER-Lung 3 [NCT03409614]) examined cemiplimab-rwlc plus platinum-doublet chemotherapy in 466 patients with stage III or IV advanced NSCLC. Patients had not received previous chemotherapy and had no EGFR, ALK, or ROS1 genomic tumor aberrations. Patients were randomly assigned (2:1) to receive cemiplimab-rwlc 350 mg (n = 312) or placebo (n = 154) every 3 weeks for up to 108 weeks along with four cycles of platinum-doublet chemotherapy. Patients also received pemetrexed maintenance as indicated. The primary end point was OS. The trial met preset OS efficacy criteria and was stopped early on the recommendation of the independent data monitoring committee.[91][Level of evidence A1]
- The median OS was 21.9 months (95% CI, 15.5–not evaluable [NE]) in the cemiplimab-rwlc-plus-chemotherapy group and 13 months (95% CI, 11.9–16.1) in the placebo-plus-chemotherapy group (HR, 0.71; 95% CI, 0.53–0.93; P = .014).
- The secondary end point of median PFS was 8.2 months (95% CI, 6.4–9.3) in the cemiplimab-rwlc-plus-chemotherapy group and 5.0 months (95% CI, 4.3–6.2) in the placebo-plus-chemotherapy group (HR, 0.56; 95% CI, 0.44–0.70; P < .0001).
- Another secondary end point, the estimated proportion of patients alive at 12 months, was 65.7% (95% CI, 59.9%–70.9%) in the cemiplimab-rwlc-plus-chemotherapy group and 56.1% (95% CI, 47.5%–63.8%) in the placebo-plus-chemotherapy group.
- Grade 3 or greater adverse events occurred in 43.6% (136 of 312) of patients who received cemiplimab-rwlc plus chemotherapy and 31.4% (48 of 153) of patients who received placebo plus chemotherapy.
- The most common (≥15%) adverse reactions were alopecia, musculoskeletal pain, nausea, fatigue, peripheral neuropathy, and decreased appetite.
The FDA approved cemiplimab-rwlc in combination with platinum-based chemotherapy for adult patients with advanced NSCLC and no EGFR, ALK, or ROS1 aberrations.
Cemiplimab-rwlc alone
Evidence (cemiplimab-rwlc alone):
- A phase III open-label study (EMPOWER-Lung 1 [NCT03088540]) enrolled 710 patients with advanced NSCLC and PD-L1 tumor expression of at least 50%. Patients were randomly assigned (1:1) to receive cemiplimab-rwlc 350mg every 3 weeks for up to 108 weeks or platinum-doublet chemotherapy. Patients could cross over from chemotherapy to cemiplimab-rwlc in the event of disease progression. There was also the option to cross over to continue cemiplimab-rwlc plus four cycles of chemotherapy in the event of progression with cemiplimab alone. Primary end points were OS and PFS per blinded independent central review. The median follow-up was 37 months for the ITT population.[92,93][Level of evidence A1]
- At 35 months of follow-up, in patients with PD-L1 expression ≥50%, the median OS was 26.1 months with cemiplimab-rwlc and 13.3 months with chemotherapy (HR, 0.57; P < .0001).
- The median PFS was 8.1 months for patients who received cemiplimab-rwlc and 5.3 months for patients who received chemotherapy (HR, 0.51; P < .0001).
- The objective response rate was 46% for patients who received cemiplimab-rwlc and 21% for patients who received chemotherapy (OR, 3.264; P < .0001).
- Benefits were greater in patients with PD-L1 expression ≥90% versus 50% to 89%.
- Among 64 patients who received cemiplimab-rwlc plus chemotherapy after initial progression on cemiplimab-rwlc alone, the median PFS was 6.6 months, the objective response rate was 31%, and the OS was 15.1 months.
- The most common adverse reactions (>10%) with cemiplimab-rwlc were musculoskeletal pain, rash, anemia, fatigue, decreased appetite, pneumonia, and cough. The safety profile of cemiplimab-rwlc was consistent over longer follow-up.
The FDA approved cemiplimab-rwlc for patients with advanced NSCLC (locally advanced who are not candidates for surgical resection or definitive chemoradiation or metastatic) and PD-L1 tumor expression of at least 50% with no EGFR, anaplastic ALK, or ROS1 genomic aberrations.
Tremelimumab
Tremelimumab is a fully human monoclonal antibody against cytotoxic T-lymphocyte associated antigen 4 (CTLA-4). It is an immune checkpoint blocker.
Durvalumab plus tremelimumab plus chemotherapy
Evidence (durvalumab plus tremelimumab plus chemotherapy):
- POSEIDON (NCT03164616), a phase III open-label trial, studied tremelimumab plus durvalumab and chemotherapy, durvalumab plus chemotherapy, and chemotherapy alone as first-line therapy in patients with metastatic NSCLC. The primary end points were PFS and OS for durvalumab plus chemotherapy versus chemotherapy. Key alpha-controlled secondary end points were PFS and OS for tremelimumab plus durvalumab and chemotherapy versus chemotherapy. Patients were randomly assigned (1:1:1) to one of the following three arms:[94][Level of evidence B1]
- Arm 1: Tremelimumab 75 mg plus durvalumab 1,500 mg with platinum-based chemotherapy for up to four 21-day cycles followed by durvalumab once every 4 weeks until progression and one additional tremelimumab dose at week 16.
- Arm 2: Durvalumab plus chemotherapy for up to four 21-day cycles followed by durvalumab once every 4 weeks until progression.
- Arm 3: Platinum-based chemotherapy for up to six 21-day cycles (with or without maintenance pemetrexed).
The following results were observed:
- Treatment with durvalumab significantly improved PFS compared with chemotherapy alone. The median PFS was 5.5 months for patients who received durvalumab plus chemotherapy and 4.8 months for patients who received chemotherapy alone (HR, 0.74; 95% CI, 0.62–0.89; P = .0009).
- A trend for improved OS did not reach statistical significance for patients in arms 2 and 3. The median OS was 13.3 months for patients who received durvalumab plus chemotherapy and 11.7 months for patients who received chemotherapy alone. (HR, 0.86; 95% CI, 0.72–1.02; P = .0758). The 24-month OS rate was 29.6% in the durvalumab-plus-chemotherapy arm and 22.1% in the chemotherapy-alone arm.
- Both PFS and OS were significantly improved when tremelimumab therapy was added to durvalumab and chemotherapy compared with chemotherapy alone. The median PFS was 6.2 months for patients who received tremelimumab plus durvalumab and chemotherapy and 4.8 months for patients who received chemotherapy alone (HR, 0.72; 95% CI, 0.60–0.86; P = .0003). The median OS was 14.0 months in the tremelimumab arm and 11.7 months in the chemotherapy-alone arm (HR, 0.77; 95% CI, 0.65–0.92; P = .0030). The 24-month OS rate was 32.9% in the tremelimumab-plus durvalumab-and-chemotherapy arm and 22.1% in the chemotherapy-alone arm. In addition, this combination demonstrated consistent OS results across levels of PD-L1 expression.
- Grade 3 to 4 treatment-related events occurred in 51.8% of patients who received tremelimumab plus durvalumab and chemotherapy, 14.1% of patients who received durvalumab and chemotherapy, and 9.9% of patients who received chemotherapy alone.
The FDA approved tremelimumab in combination with durvalumab and platinum-based chemotherapy for adult patients with metastatic NSCLC with no sensitizing EGFR or ALK genomic tumor aberrations. The approval is based on a comparison of treatment arms one and three.
Atezolizumab alone
Evidence (atezolizumab alone):
- A phase III open-label study (IMpower110 [NCT02409342]) included 572 patients with previously untreated metastatic nonsquamous or squamous NSCLC. Patients had PD-L1 expression on at least 1% of tumor cells or on at least 1% of tumor-infiltrating immune cells. Patients were randomly assigned to receive either atezolizumab (1,200 mg intravenously) or platinum-based chemotherapy (4 or 6 cycles) once every 3 weeks. The primary end point was OS in the PD-L1–selected population that excluded sensitizing EGFR mutations or ALK translocations.[95][Level of evidence A1]
- PD-L1 expression was assessed by the SP142 immunohistochemical assay. High expression was defined as more than 50% of tumor cells or more than 10% of tumor-infiltrating immune cells expressing PD-L1.
- In the 205 patients with high PD-L1 expression, the median OS was 20.2 months for patients who received atezolizumab and 13.1 months for patients who received chemotherapy (HRdeath, 0.59; P = .01).
- Grade 3 to 4 adverse events occurred in 30.1% of patients who received atezolizumab and 52.5% of patients who received chemotherapy.
Atezolizumab monotherapy is approved for first-line treatment of patients with high PD-L1 expression (PD-L1 staining ≥50% of tumor cells or PD-L1 stained tumor-infiltrating immune cells covering ≥10% of the tumor area), as determined by an FDA-approved test, in the absence of EGFR or ALK genomic aberrations.
Atezolizumab plus chemotherapy
Evidence (atezolizumab in combination with carboplatin and nab-paclitaxel chemotherapy):
- A phase III open-label study (IMpower130 [NCT02367781]) included 724 patients with previously untreated, stage IV, nonsquamous NSCLC. Patients were randomly assigned 2:1 to receive atezolizumab (1,200 mg intravenously every 3 weeks) plus chemotherapy (carboplatin, AUC 6 mg/mL per minute every 3 weeks with nab-paclitaxel 100 mg/m2 intravenously every week), or chemotherapy alone given once every 3 weeks for four or six cycles. All patients received maintenance therapy as follows: (1) patients in the atezolizumab-plus-chemotherapy group received atezolizumab 1,200 mg intravenously every 3 weeks until investigator-assessed loss of clinical benefit or toxicity, and (2) patients in the chemotherapy-alone group received best supportive care or pemetrexed switch maintenance therapy until disease progression or toxicity. Coprimary end points were investigator-assessed PFS and OS in the ITT population with EGFR wild-type and ALK wild-type tumors.[96][Level of evidence A1]
- In the ITT wild-type population, the median OS was 18.6 months (95% CI, 16.0–21.2) in the atezolizumab-plus-chemotherapy group and 13.9 months (95% CI, 12.0–18.7) in the chemotherapy group (stratified HR, 0.79; 95% CI, 0.64–0.98; P = .033).
- The median PFS was 7 months (95% CI, 6.2–7.3) in the atezolizumab-plus-chemotherapy group and 5.5 months (95% CI, 4.4–5.9) in the chemotherapy group (stratified HR, 0.64; 95% CI, 0.54–0.77; P < .0001).
- Subgroup analyses showed OS and PFS benefit with atezolizumab across several clinical subgroups, with the exception of patients with liver metastases where the additional of atezolizumab did not improve OS versus chemotherapy alone, and for patients with EGFR and ALK genomic alterations.
- OS and PFS benefit with atezolizumab was also observed in the ITT wild-type population independent of PD-L1 expression.
- Grade 3 or 4 adverse events occurred in 81% of patients who received atezolizumab plus chemotherapy versus 71% of patients who received chemotherapy alone. Immune-related adverse events occurred in 45% of patients treated with atezolizumab plus chemotherapy and most were grade 1 or 2 in severity. The most common immune-related adverse events were rash (24%), hypothyroidism (15%), and hepatitis (10%).
Atezolizumab in combination with nab-paclitaxel and carboplatin is approved for the first-line treatment of patients with metastatic nonsquamous NSCLC with no EGFR or ALK genomic aberrations.
Atezolizumab plus bevacizumab plus chemotherapy
Evidence (atezolizumab in combination with carboplatin, paclitaxel, and bevacizumab):
- In a phase III open-label study (IMpower150 [NCT02366143]),1,202 patients with stage IV or recurrent metastatic nonsquamous NSCLC were randomly assigned in a 1:1:1 ratio to receive either atezolizumab plus carboplatin plus paclitaxel (ACP group), atezolizumab plus bevacizumab plus carboplatin plus paclitaxel (ABCP group), or bevacizumab plus carboplatin plus paclitaxel (BCP group). Treatment consisted of four or six 21-day cycles. Atezolizumab was given intravenously at a dose of 1,200 mg, bevacizumab at a dose of 15 mg per kilogram of body weight, carboplatin at an area under the concentration-time curve of 6 mg/mL per minute and paclitaxel at a dose of 200 mg/m2 (175 mg/m2 for Asian patients). Patients continued to receive atezolizumab, bevacizumab, or both until disease progression or development of intolerable toxicity. Coprimary end points were PFS, both in the ITT population with EGFR wild-type and ALK wild-type tumors and among patients with wild-type tumors who had high expression of an effector T-cell (Teff) gene signature in the tumor, and OS in the wild-type population.[97][Level of evidence A1]
- Median PFS was longer in the ABCP group (8.3 months) than the BCP group (6.8 months) (HR, 0.62; 95% CI, 0.52–0.74; P < .001). In the Teff-high wild-type population, PFS was 11.3 months versus 6.8 months (HR, 0.51; 95% CI, 0.38–0.68; P .001). PFS was also longer in the ABCP group versus the BCP group in the ITT population with EGFR and ALK genomic alterations, among patients with low or negative PD-L1 expression, low Teff gene-signature expression, and in patients with liver metastases.
- Median OS among patients with wild-type tumors was longer in the ABCP group (19.2 months), compared with the BCP group (14.7 months) (HR, 0.78; 95% CI, 0.64–0.96; P = .02).
- Grade 3 or 4 treatment-related adverse events occurred in 56% of patients in the ABCP group versus 48% of patients in the BCP group. Most immune-related adverse events in the ABCP group were grade 1 or 2, and rash, hypothyroidism, hyperthyroidism, hepatitis, pneumonitis, and colitis were most common. Treatment-related deaths occurred in 11 patients (2.8%) in the ABCP group and 9 patients (2.3%) in the BCP group. Five deaths in the ABCP group were caused by pulmonary hemorrhage or hemoptysis, and four of five occurred in patients with high-risk features, including tumors infiltrating great vessels or tumor cavitation.
Atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin is approved for the first-line treatment of patients with metastatic nonsquamous NSCLC with no EGFR or ALK genomic aberrations.
Nivolumab plus ipilimumab
Nivolumab, a fully human anti–PD-1 antibody, and ipilimumab, a fully human anti–CTLA-4 antibody, are immune checkpoint inhibitors with distinct but complementary mechanisms of action.[98]
Evidence (nivolumab plus ipilimumab):
- A phase III open-label study (CheckMate 227 [NCT02477826]) evaluated nivolumab in combination with ipilimumab versus chemotherapy as first-line treatment for stage IV or recurrent NSCLC without sensitizing EGFR mutations or ALK translocations. Patients (n = 1,739) were grouped by PD-L1 tumor status (either ≥1% or <1%). Patients with PD-L1 expression of at least 1% were randomly assigned to receive either nivolumab (3 mg/kg every 2 weeks) plus ipilimumab (1 mg/kg every 6 weeks), nivolumab (240 mg every 2 weeks) alone, or platinum-doublet chemotherapy every 3 weeks for up to four cycles. Patients with PD-L1 expression less than 1% were randomly assigned to receive nivolumab with ipilimumab, nivolumab with platinum-doublet chemotherapy, or platinum-doublet chemotherapy (every 3 weeks). Patients were treated until disease progression or unacceptable toxicity or up to 2 years for immunotherapy. Coprimary end points were OS with nivolumab-plus-ipilimumab compared with chemotherapy in patients with tumor PD-L1 expression of at least 1%, and PFS with nivolumab-plus-ipilimumab compared with chemotherapy in patients with high tumor mutational burden (TMB) (≥10 mutations per megabase).[2,99][Level of evidence A1]
- Among patients with tumor PD-L1 ≥1% (n = 1,189), the median OS was 17.1 months (95% CI, 15.0–20.2) with nivolumab-plus-ipilimumab and 14.9 months (95% CI, 12.7–16.7) with chemotherapy (HR, 0.77; 95% CI, 0.66–0.91; P = .007). Five-year outcomes with nivolumab-plus-ipilimumab versus chemotherapy showed durable clinical benefit, with an OS rate of 24% with nivolumab-plus-ipilimumab and 14% for chemotherapy alone.[98]
- In patients with TMB-high NSCLC, the median PFS was 7.2 months (95% CI, 5.5–13.2) with nivolumab-plus-ipilimumab, versus 5.5 months (95% CI, 4.4–5.8) with chemotherapy alone (HR, 0.58; 97.5% CI, 0.41–0.81; P < .001).
- Among patients with tumor PD-L1 <1% (n = 550), the median OS was 17.4 months (95% CI, 13.2–22.0) with nivolumab-plus-ipilimumab, and 12.2 months (95% CI, 9.2–14.3) with chemotherapy alone (HR, 0.65; 95% CI, 0.52–0.81). Five-year outcomes with nivolumab-plus-ipilimumab versus chemotherapy alone showed durable clinical benefit, with an OS rate of 19% for nivolumab-plus-ipilimumab and 7% for chemotherapy alone.[98]
- The frequency of grade 3 to 4 treatment-related adverse events was similar in both groups (32.8% with nivolumab-plus-ipilimumab vs. 36.0% with chemotherapy alone). Treatment-related adverse events leading to therapy discontinuation were more common with nivolumab-plus-ipilimumab than with chemotherapy alone (24.5% vs. 13.9%).
- Treatment-related deaths occurred in eight patients who received nivolumab-plus-ipilimumab (pneumonitis in four patients; shock, myocarditis, acute tubular necrosis, and cardiac tamponade in one patient each) and in six patients who received chemotherapy (sepsis in two patients; febrile neutropenia, multifocal brain infarctions, interstitial lung disease, and thrombocytopenia in one patient each).
The FDA approved nivolumab-plus-ipilimumab as ï¬rst-line therapy for patients with advanced NSCLC with PD-L1 expression of at least 1% and no EGFR or ALK genomic aberrations. While this regimen is not FDA-approved for patients with PD-L1 expression less than 1%, these patients were noted to have durable clinical benefit in CheckMate 227.
mTOR inhibitors
Everolimus
Everolimus is used for patients with unresectable, locally advanced or metastatic, progressive, well-differentiated, nonfunctional, neuroendocrine tumors.
Everolimus, an oral mTOR inhibitor, is clinically active against advanced pancreatic and nonpancreatic neuroendocrine tumors.[100] Based on the results of the RADIANT-4 clinical trial,[100] the FDA approved everolimus for the treatment of adult patients with unresectable, locally advanced or metastatic, progressive, well-differentiated (low or intermediate grade), nonfunctional neuroendocrine tumors of lung or gastrointestinal origin.
Evidence (everolimus):
- A randomized, double-blind, placebo-controlled, phase III trial (RADIANT-4 [NCT01524783]) evaluated everolimus in patients older than 18 years with advanced, progressive, well-differentiated, nonfunctional neuroendocrine tumors of lung or gastrointestinal origin.[100] Eligible patients were randomly assigned in a 2:1 ratio to received everolimus 10 mg daily orally or placebo, both with best supportive care. A total of 302 patients were enrolled (205 in the everolimus arm and 97 in the placebo arm), including 90 patients with neuroendocrine tumors of lung origin (63 in the everolimus arm and 27 in the placebo arm). The primary end point was PFS assessed by central radiology review in the ITT population.
- Median PFS was 11.0 months in the everolimus arm and 3.9 months in the placebo group (HR, 0.48; 95% CI, 0.35−0.67; P < .00001).[100][Level of evidence B1]
- In a post hoc analysis of the lung subgroup, median PFS by central review was 9.2 months in the everolimus arm and 3.6 months in the placebo arm (HR, 0.50; 95% CI, 0.28−0.88).[100]
- The objective response rate was 2% in patients who received everolimus and 1% in patients who received placebo. Disease stabilization was observed in 81% of patients in the everolimus arm and 64% of patients in the placebo arm.
- The median duration of treatment was longer in the patients who received everolimus compared with those who received placebo (40.4 weeks vs. 19.6 weeks).
- A planned interim analysis of OS showed a 36% reduction in the estimated risk of death with everolimus relative to placebo (HR, 0.64; 95% CI, 0.40−1.05). These results were not statistically significant.
- The most common treatment-related adverse events were stomatitis, diarrhea, fatigue, infections, rash, and peripheral edema. The most common drug-related grade 3 or 4 adverse events were stomatitis, diarrhea, infections, anemia, and fatigue. Grade 3 or 4 adverse events resulted in treatment discontinuation in 12% of patients in the everolimus group and 3% of patients in the placebo group.
Local therapies and special considerations
Endobronchial laser therapy and/or brachytherapy (for obstruction lesions)
Radiation therapy may be effective in palliating symptomatic patients with local involvement of NSCLC with any of the following:
- Tracheal, esophageal, or bronchial compression.
- Pain.
- Vocal cord paralysis.
- Hemoptysis.
- Superior vena cava syndrome.
In some cases, endobronchial laser therapy and/or brachytherapy have been used to alleviate proximal obstructing lesions.[20]
EBRT (primarily for palliation of local symptomatic tumor growth)
Although EBRT is frequently prescribed for symptom palliation, there is no consensus on which fractionation scheme should be used. Although different multifraction regimens appear to provide similar symptom relief,[101,102,103,104,105,106] single-fraction radiation may be insufficient for symptom relief compared with hypofractionated or standard regimens, as evidenced in the NCT00003685 trial.[21][Level of evidence A3] Evidence of a modest increase in survival in patients with a better performance status given high-dose radiation therapy is available.[23,107][Level of evidence A1] In closely observed asymptomatic patients, treatment may often be appropriately deferred until symptoms or signs of a progressive tumor develop.
Evidence (radiation therapy):
- A systematic review identified six randomized trials of high-dose rate endobronchial brachytherapy (HDREB) alone or with EBRT or laser therapy.[108]
- Better overall symptom palliation and fewer re-treatments were required in previously untreated patients using EBRT alone.[108][Level of evidence A3]
- HDREB provided palliation of symptomatic patients with recurrent endobronchial obstruction previously treated by EBRT, when it was technically feasible.
Treatment of second primary tumor
A solitary pulmonary metastasis from an initially resected bronchogenic carcinoma is unusual. The lung is frequently the site of second primary malignancies in patients with primary lung cancers. Whether the new lesion is a new primary cancer or a metastasis may be difficult to determine. Studies have indicated that in most patients the new lesion is a second primary tumor, and after its resection, some patients may achieve long-term survival. Thus, if the first primary tumor has been controlled, the second primary tumor should be resected, if possible.[109,110]
Treatment of brain metastases
Patients who present with a solitary cerebral metastasis after resection of a primary NSCLC lesion and who have no evidence of extracranial tumor can achieve prolonged disease-free survival with surgical excision of the brain metastasis and postoperative whole-brain radiation therapy.[111,112] Unresectable brain metastases in this setting may be treated with stereotactic radiosurgery.[113]
Approximately 50% of patients treated with resection and postoperative radiation therapy will develop recurrence in the brain; some of these patients will be suitable for additional treatment.[114] In those selected patients with good performance status and without progressive metastases outside of the brain, treatment options include reoperation or stereotactic radiation surgery.[113,114] For most patients, additional radiation therapy can be considered; however, the palliative benefit of this treatment is limited.[115][Level of evidence C2]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Gandhi L, RodrÃguez-Abreu D, Gadgeel S, et al.: Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 378 (22): 2078-2092, 2018.
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al.: Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 381 (21): 2020-2031, 2019.
- Weick JK, Crowley J, Natale RB, et al.: A randomized trial of five cisplatin-containing treatments in patients with metastatic non-small-cell lung cancer: a Southwest Oncology Group study. J Clin Oncol 9 (7): 1157-62, 1991.
- Scagliotti GV, Parikh P, von Pawel J, et al.: Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26 (21): 3543-51, 2008.
- Langer CJ, Vangel M, Schiller J, et al.: Age-specific subanalysis of ECOG 1594: fit elderly patients (70-80 YRS) with NSCLC do as well as younger pts (<70). [Abstract] Proceedings of the American Society of Clinical Oncology 22: A-2571, 2003.
- Langer CJ, Manola J, Bernardo P, et al.: Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst 94 (3): 173-81, 2002.
- Ries LA: Influence of extent of disease, histology, and demographic factors on lung cancer survival in the SEER population-based data. Semin Surg Oncol 10 (1): 21-30, 1994 Jan-Feb.
- Ramsey SD, Howlader N, Etzioni RD, et al.: Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: evidence from surveillance, epidemiology and end results-Medicare. J Clin Oncol 22 (24): 4971-8, 2004.
- Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J Natl Cancer Inst 91 (1): 66-72, 1999.
- Takeda K, Kudoh S, Nakagawa K, et al.: Randomized phase III study of docetaxel (D) versus vinorelbine (V) for elderly patients (pts) with advanced non-small cell lung cancer (NSCLC): Results of a West Japan Thoracic Oncology Group trial (WJTOG9904). [Abstract] J Clin Oncol 23 (Suppl 16): A-7009, 2005.
- Schiller JH, Harrington D, Belani CP, et al.: Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346 (2): 92-8, 2002.
- Belani CP, Fossella F: Elderly subgroup analysis of a randomized phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for first-line treatment of advanced nonsmall cell lung carcinoma (TAX 326). Cancer 104 (12): 2766-74, 2005.
- Lilenbaum RC, Herndon JE, List MA, et al.: Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 23 (1): 190-6, 2005.
- Hensing TA, Peterman AH, Schell MJ, et al.: The impact of age on toxicity, response rate, quality of life, and survival in patients with advanced, Stage IIIB or IV nonsmall cell lung carcinoma treated with carboplatin and paclitaxel. Cancer 98 (4): 779-88, 2003.
- Sweeney CJ, Zhu J, Sandler AB, et al.: Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a Phase II trial in patients with metastatic nonsmall cell lung carcinoma . Cancer 92 (10): 2639-47, 2001.
- Zukin M, Barrios CH, Pereira JR, et al.: Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 31 (23): 2849-53, 2013.
- Tester WJ, Stephenson P, Langer CJ, et al.: ECOG 1599: randomized phase II study of paclitaxel/carboplatin or gemcitabine/cisplatin in performance status (PS) 2 patients with advanced non-small cell lung cancer (NSCLC). [Abstract] J Clin Oncol 22 (Suppl 14): A-7055, 630s, 2004.
- Vansteenkiste JF, Vandebroek JE, Nackaerts KL, et al.: Clinical-benefit response in advanced non-small-cell lung cancer: A multicentre prospective randomised phase III study of single agent gemcitabine versus cisplatin-vindesine. Ann Oncol 12 (9): 1221-30, 2001.
- Hickish TF, Smith IE, O'Brien ME, et al.: Clinical benefit from palliative chemotherapy in non-small-cell lung cancer extends to the elderly and those with poor prognostic factors. Br J Cancer 78 (1): 28-33, 1998.
- Miller JI, Phillips TW: Neodymium:YAG laser and brachytherapy in the management of inoperable bronchogenic carcinoma. Ann Thorac Surg 50 (2): 190-5; discussion 195-6, 1990.
- Bezjak A, Dixon P, Brundage M, et al.: Randomized phase III trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (NCIC CTG SC.15). Int J Radiat Oncol Biol Phys 54 (3): 719-28, 2002.
- Macbeth F, Toy E, Coles B, et al.: Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev (3): CD002143, 2001.
- Sundstrøm S, Bremnes R, Aasebø U, et al.: Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol 22 (5): 801-10, 2004.
- Delbaldo C, Michiels S, Rolland E, et al.: Second or third additional chemotherapy drug for non-small cell lung cancer in patients with advanced disease. Cochrane Database Syst Rev (4): CD004569, 2007.
- Ardizzoni A, Boni L, Tiseo M, et al.: Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 99 (11): 847-57, 2007.
- Jiang J, Liang X, Zhou X, et al.: A meta-analysis of randomized controlled trials comparing carboplatin-based to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer 57 (3): 348-58, 2007.
- Hotta K, Matsuo K, Ueoka H, et al.: Meta-analysis of randomized clinical trials comparing Cisplatin to Carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 22 (19): 3852-9, 2004.
- D'Addario G, Pintilie M, Leighl NB, et al.: Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol 23 (13): 2926-36, 2005.
- Rajeswaran A, Trojan A, Burnand B, et al.: Efficacy and side effects of cisplatin- and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: a systematic review of randomized controlled trials. Lung Cancer 59 (1): 1-11, 2008.
- Pujol JL, Barlesi F, Daurès JP: Should chemotherapy combinations for advanced non-small cell lung cancer be platinum-based? A meta-analysis of phase III randomized trials. Lung Cancer 51 (3): 335-45, 2006.
- Douillard JY, Laporte S, Fossella F, et al.: Comparison of docetaxel- and vinca alkaloid-based chemotherapy in the first-line treatment of advanced non-small cell lung cancer: a meta-analysis of seven randomized clinical trials. J Thorac Oncol 2 (10): 939-46, 2007.
- Belani CP, Ramalingam S, Perry MC, et al.: Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J Clin Oncol 26 (3): 468-73, 2008.
- Schuette W, Blankenburg T, Guschall W, et al.: Multicenter randomized trial for stage IIIB/IV non-small-cell lung cancer using every-3-week versus weekly paclitaxel/carboplatin. Clin Lung Cancer 7 (5): 338-43, 2006.
- Sandler A, Gray R, Perry MC, et al.: Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355 (24): 2542-50, 2006.
- Reck M, von Pawel J, Zatloukal P, et al.: Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27 (8): 1227-34, 2009.
- Lynch TJ, Patel T, Dreisbach L, et al.: Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 28 (6): 911-7, 2010.
- Pirker R, Pereira JR, Szczesna A, et al.: Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 373 (9674): 1525-31, 2009.
- Khambata-Ford S, Harbison CT, Hart LL, et al.: Analysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 28 (6): 918-27, 2010.
- Paz-Ares L, Mezger J, Ciuleanu TE, et al.: Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol 16 (3): 328-37, 2015.
- Thatcher N, Hirsch FR, Luft AV, et al.: Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 16 (7): 763-74, 2015.
- Paz-Ares LG, de Marinis F, Dediu M, et al.: PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 31 (23): 2895-902, 2013.
- Brodowicz T, Krzakowski M, Zwitter M, et al.: Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer 52 (2): 155-63, 2006.
- Park JO, Kim SW, Ahn JS, et al.: Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non small-cell lung cancer. J Clin Oncol 25 (33): 5233-9, 2007.
- Paz-Ares L, de Marinis F, Dediu M, et al.: Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 13 (3): 247-55, 2012.
- Socinski MA, Schell MJ, Peterman A, et al.: Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol 20 (5): 1335-43, 2002.
- von Plessen C, Bergman B, Andresen O, et al.: Palliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancer. Br J Cancer 95 (8): 966-73, 2006.
- Ciuleanu T, Brodowicz T, Zielinski C, et al.: Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374 (9699): 1432-40, 2009.
- Belani CP, Brodowicz T, Ciuleanu TE, et al.: Quality of life in patients with advanced non-small-cell lung cancer given maintenance treatment with pemetrexed versus placebo (H3E-MC-JMEN): results from a randomised, double-blind, phase 3 study. Lancet Oncol 13 (3): 292-9, 2012.
- Soria JC, Ohe Y, Vansteenkiste J, et al.: Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 378 (2): 113-125, 2018.
- Ramalingam SS, Vansteenkiste J, Planchard D, et al.: Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 382 (1): 41-50, 2020.
- Planchard D, Jänne PA, Cheng Y, et al.: Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N Engl J Med 389 (21): 1935-1948, 2023.
- Wu YL, Cheng Y, Zhou X, et al.: Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 18 (11): 1454-1466, 2017.
- Mok TS, Wu YL, Thongprasert S, et al.: Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361 (10): 947-57, 2009.
- Maemondo M, Inoue A, Kobayashi K, et al.: Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362 (25): 2380-8, 2010.
- Mitsudomi T, Morita S, Yatabe Y, et al.: Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11 (2): 121-8, 2010.
- Zhou C, Wu YL, Chen G, et al.: Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12 (8): 735-42, 2011.
- Rosell R, Gervais R, Vergnenegre A, et al.: Erlotinib versus chemotherapy (CT) in advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: Interim results of the European Erlotinib Versus Chemotherapy (EURTAC) phase III randomized trial. [Abstract] J Clin Oncol 29 (Suppl 15): A-7503, 2011.
- Rosell R, Carcereny E, Gervais R, et al.: Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13 (3): 239-46, 2012.
- Sequist LV, Yang JC, Yamamoto N, et al.: Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31 (27): 3327-34, 2013.
- Yang JC, Wu YL, Schuler M, et al.: Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16 (2): 141-51, 2015.
- Wu YL, Zhou C, Hu CP, et al.: Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15 (2): 213-22, 2014.
- Zhou C, Tang KJ, Cho BC, et al.: Amivantamab plus Chemotherapy in NSCLC with EGFR Exon 20 Insertions. N Engl J Med 389 (22): 2039-2051, 2023.
- Peters S, Camidge DR, Shaw AT, et al.: Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 377 (9): 829-838, 2017.
- Hida T, Nokihara H, Kondo M, et al.: Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 390 (10089): 29-39, 2017.
- Shaw AT, Bauer TM, de Marinis F, et al.: First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 383 (21): 2018-2029, 2020.
- Solomon BJ, Bauer TM, Ignatius Ou SH, et al.: Post Hoc Analysis of Lorlatinib Intracranial Efficacy and Safety in Patients With ALK-Positive Advanced Non-Small-Cell Lung Cancer From the Phase III CROWN Study. J Clin Oncol 40 (31): 3593-3602, 2022.
- Solomon BJ, Mok T, Kim DW, et al.: First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371 (23): 2167-77, 2014.
- Mok T, Kim D, Wu Y, et al.: First-line crizotinib versus pemetrexed-cisplatin or pemetrexed-carboplatin in patients (pts) with advanced ALK-positive non-squamous non-small cell lung cancer (NSCLC): results of a phase III study (PROFILE 1014). [Abstract] J Clin Oncol 32 (Suppl 15): A-8002, 2014.
- Soria JC, Tan DSW, Chiari R, et al.: First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 389 (10072): 917-929, 2017.
- Kim DW, Tiseo M, Ahn MJ, et al.: Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 35 (22): 2490-2498, 2017.
- Planchard D, Smit EF, Groen HJM, et al.: Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 18 (10): 1307-1316, 2017.
- Gainor JF, Shaw AT: Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 18 (7): 865-75, 2013.
- Drilon A, Siena S, Dziadziuszko R, et al.: Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol 21 (2): 261-270, 2020.
- Shaw AT, Ou SH, Bang YJ, et al.: Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 371 (21): 1963-71, 2014.
- Wu YL, Yang JC, Kim DW, et al.: Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 36 (14): 1405-1411, 2018.
- Farago AF, Le LP, Zheng Z, et al.: Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 10 (12): 1670-4, 2015.
- Gatalica Z, Xiu J, Swensen J, et al.: Molecular characterization of cancers with NTRK gene fusions. Mod Pathol 32 (1): 147-153, 2019.
- Drilon A, Laetsch TW, Kummar S, et al.: Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 378 (8): 731-739, 2018.
- Doebele RC, Drilon A, Paz-Ares L, et al.: Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 21 (2): 271-282, 2020.
- Drilon A, Hu ZI, Lai GGY, et al.: Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 15 (3): 151-167, 2018.
- Drilon A, Subbiah V, Gautschi O, et al.: Selpercatinib in Patients With RET Fusion-Positive Non-Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. J Clin Oncol 41 (2): 385-394, 2023.
- Gainor JF, Curigliano G, Kim DW, et al.: Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol 22 (7): 959-969, 2021.
- Awad MM, Oxnard GR, Jackman DM, et al.: MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J Clin Oncol 34 (7): 721-30, 2016.
- Paik PK, Felip E, Veillon R, et al.: Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 383 (10): 931-943, 2020.
- Wolf J, Seto T, Han JY, et al.: Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med 383 (10): 944-957, 2020.
- Reck M, RodrÃguez-Abreu D, Robinson AG, et al.: Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375 (19): 1823-1833, 2016.
- Garassino MC, Gadgeel S, Speranza G, et al.: Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol 41 (11): 1992-1998, 2023.
- Novello S, Kowalski DM, Luft A, et al.: Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J Clin Oncol 41 (11): 1999-2006, 2023.
- Reck M, RodrÃguez-Abreu D, Robinson AG, et al.: Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 37 (7): 537-546, 2019.
- de Castro G, Kudaba I, Wu YL, et al.: Five-Year Outcomes With Pembrolizumab Versus Chemotherapy as First-Line Therapy in Patients With Non-Small-Cell Lung Cancer and Programmed Death Ligand-1 Tumor Proportion Score ≥ 1% in the KEYNOTE-042 Study. J Clin Oncol 41 (11): 1986-1991, 2023.
- Gogishvili M, Melkadze T, Makharadze T, et al.: Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med 28 (11): 2374-2380, 2022.
- Sezer A, Kilickap S, Gümüş M, et al.: Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 397 (10274): 592-604, 2021.
- Özgüroğlu M, Kilickap S, Sezer A, et al.: First-line cemiplimab monotherapy and continued cemiplimab beyond progression plus chemotherapy for advanced non-small-cell lung cancer with PD-L1 50% or more (EMPOWER-Lung 1): 35-month follow-up from a mutlicentre, open-label, randomised, phase 3 trial. Lancet Oncol 24 (9): 989-1001, 2023.
- Johnson ML, Cho BC, Luft A, et al.: Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J Clin Oncol 41 (6): 1213-1227, 2023.
- Herbst RS, Giaccone G, de Marinis F, et al.: Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 383 (14): 1328-1339, 2020.
- West H, McCleod M, Hussein M, et al.: Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20 (7): 924-937, 2019.
- Socinski MA, Jotte RM, Cappuzzo F, et al.: Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 378 (24): 2288-2301, 2018.
- Brahmer JR, Lee JS, Ciuleanu TE, et al.: Five-Year Survival Outcomes With Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227. J Clin Oncol 41 (6): 1200-1212, 2023.
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al.: Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 378 (22): 2093-2104, 2018.
- Yao JC, Fazio N, Singh S, et al.: Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387 (10022): 968-977, 2016.
- Danson S, Middleton MR, O'Byrne KJ, et al.: Phase III trial of gemcitabine and carboplatin versus mitomycin, ifosfamide, and cisplatin or mitomycin, vinblastine, and cisplatin in patients with advanced nonsmall cell lung carcinoma. Cancer 98 (3): 542-53, 2003.
- Pfister DG, Johnson DH, Azzoli CG, et al.: American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 22 (2): 330-53, 2004.
- Smit EF, van Meerbeeck JP, Lianes P, et al.: Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group--EORTC 08975. J Clin Oncol 21 (21): 3909-17, 2003.
- Kubota K, Watanabe K, Kunitoh H, et al.: Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: the Japanese Taxotere Lung Cancer Study Group. J Clin Oncol 22 (2): 254-61, 2004.
- Georgoulias V, Ardavanis A, Agelidou A, et al.: Docetaxel versus docetaxel plus cisplatin as front-line treatment of patients with advanced non-small-cell lung cancer: a randomized, multicenter phase III trial. J Clin Oncol 22 (13): 2602-9, 2004.
- Sandler AB, Nemunaitis J, Denham C, et al.: Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 18 (1): 122-30, 2000.
- Lester JF, Macbeth FR, Toy E, et al.: Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev (4): CD002143, 2006.
- Ung YC, Yu E, Falkson C, et al.: The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small-cell lung cancer: a systematic review. Brachytherapy 5 (3): 189-202, 2006 Jul-Sep.
- Salerno TA, Munro DD, Blundell PE, et al.: Second primary bronchogenic carcinoma: life-table analysis of surgical treatment. Ann Thorac Surg 27 (1): 3-6, 1979.
- Yellin A, Hill LR, Benfield JR: Bronchogenic carcinoma associated with upper aerodigestive cancers. J Thorac Cardiovasc Surg 91 (5): 674-83, 1986.
- Patchell RA, Tibbs PA, Walsh JW, et al.: A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322 (8): 494-500, 1990.
- Mandell L, Hilaris B, Sullivan M, et al.: The treatment of single brain metastasis from non-oat cell lung carcinoma. Surgery and radiation versus radiation therapy alone. Cancer 58 (3): 641-9, 1986.
- Loeffler JS, Kooy HM, Wen PY, et al.: The treatment of recurrent brain metastases with stereotactic radiosurgery. J Clin Oncol 8 (4): 576-82, 1990.
- Arbit E, Wroński M, Burt M, et al.: The treatment of patients with recurrent brain metastases. A retrospective analysis of 109 patients with nonsmall cell lung cancer. Cancer 76 (5): 765-73, 1995.
- Hazuka MB, Kinzie JJ: Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys 15 (2): 433-7, 1988.
Treatment of Progressive Stage IV, Relapsed, and Recurrent NSCLC
Treatment Options for Progressive Stage IV, Relapsed, and Recurrent NSCLC (Second-Line Therapy)
Treatment options for patients with progressive stage IV, relapsed, and recurrent non-small cell lung cancer (NSCLC) (second-line therapy and beyond) include:
- Chemotherapy.
- Epidermal growth factor receptor (EGFR)-directed therapy.
- EGFR-directed therapy after first-line chemotherapy (for patients with EGFR-sensitizing mutations).
- EGFR-directed therapy (for patients with acquired EGFR T790M mutations after previous EGFR-directed therapy).
- EGFR-directed therapy after first-line chemotherapy (for patients with EGFR exon 20 insertion mutations).
- Anaplastic lymphoma kinase (ALK)-directed tyrosine kinase inhibitors (TKIs).
- BRAF V600E and MEK inhibitors (for patients with BRAF V600E mutations).
- ROS1-directed therapy.
- Neurotrophic tyrosine kinase (NTRK) inhibitors (for patients with NTRK fusions).
- RET inhibitors (for patients with RET fusions).
- MET inhibitors (for patients with MET exon 14 skipping mutations).
- KRAS G12C inhibitors (for patients with KRAS G12C mutations).
- HER2-targeted therapy (for patients with HER2 mutations).
- Immunotherapy.
- Mammalian target of rapamycin (mTOR) inhibitors (for patients with unresectable, locally advanced or metastatic, progressive, well-differentiated, nonfunctional, neuroendocrine tumors).
- Clinical trials can be considered as second-line therapy.
Chemotherapy
The use of chemotherapy has produced objective responses and small improvement in survival for patients with metastatic disease.[1][Level of evidence A1] In studies that have examined symptomatic response, improvement in subjective symptoms has been reported to occur more frequently than objective response.[2,3] Informed patients with good performance status and symptomatic recurrence can be offered treatment with a platinum-based chemotherapy regimen for palliation of symptoms. For patients who have relapsed after platinum-based chemotherapy, second-line therapy can be considered.
Docetaxel
Evidence (docetaxel):
- Two prospective randomized studies have shown an improvement in survival with the use of docetaxel compared with vinorelbine, ifosfamide, or best supportive care;[4,5] however, criteria for the selection of appropriate patients for second-line treatment are not well defined.[6]
- A meta-analysis of five trials of 865 patients assessing the efficacy and safety of docetaxel administered weekly or every 3 weeks has been reported.[7] In that analysis, the following was shown:
- Median survival was 27.4 weeks for patients treated every 3 weeks and 26.1 weeks for patients treated weekly (log-rank P = .24).
- Significantly less severe neutropenia and febrile neutropenia were reported with weekly docetaxel (P < .001 for both); however, no significant differences were observed for anemia, thrombocytopenia, and nonhematologic toxic effects.
Docetaxel plus ramucirumab
Evidence (docetaxel plus ramucirumab):
- In a double-blind, placebo-controlled, phase III study, 1,253 patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1 who had progressive disease after first-line chemotherapy were randomly assigned to receive docetaxel and placebo or docetaxel and ramucirumab.[8][Level of evidence A1] Ramucirumab is a human immunoglobulin G1 monoclonal antibody that targets the extracellular domain of vascular endothelial growth factor receptor 2. The primary end point of the study was overall survival (OS), with secondary end points of progression-free survival (PFS) and objective response rate. The study enrolled patients with either nonsquamous or squamous NSCLC; however, patients with poorly controlled hypertension, gastrointestinal perforation or fistulae, arterial thromboembolic event within 6 months (before random assignment), gross hemoptysis within 2 months, or grade 3 to 4 gastrointestinal bleeding within 3 months were excluded. In addition, the trial did not include patients with tumors that had major blood vessel involvement or intratumor cavitation.
- The addition of ramucirumab to docetaxel compared with placebo plus docetaxel led to an increase in median OS (10.5 months vs. 9.1 months; hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.75–0.98), objective response rate (23% vs. 14%), and PFS (4.5 months vs. 3 months). The improvement in OS from the addition of ramucirumab appeared consistent across subgroups including squamous and nonsquamous histologies.
- Grade 3 to 4 treatment-related adverse events occurred in 79% of patients who received docetaxel and ramucirumab as compared with 71% of patients who received docetaxel and placebo. Febrile neutropenia, fatigue, and hypertension were among the toxicities that were more common with the addition of ramucirumab to docetaxel. There was no significant difference in the incidence of grades 3 to 4 hemorrhage between the groups.
- On the basis of this study, the addition of ramucirumab to docetaxel chemotherapy can be considered for patients with good performance status with advanced NSCLC who have progressive disease after first-line chemotherapy.
Pemetrexed
Evidence (pemetrexed):
- A randomized phase III trial of 571 patients was designed to demonstrate the noninferiority of pemetrexed compared with docetaxel.
Epidermal growth factor receptor (EGFR)-directed therapy
Advanced NSCLC that contains characteristic mutations in EGFR, most commonly exon 19 deletions or exon 21 L858R mutations, is highly sensitive to EGFR TKIs. The standard approach to decide whether to use an EGFR TKI for the treatment of a patient with advanced NSCLC is to analyze the tumor for the presence or absence of a driver mutation in EGFR. EGFR exon 20 insertions are uncommon mutations that are not sensitive to the EGFR TKIs used for the treatment of NSCLC with EGFR-sensitizing mutations.
EGFR-directed therapy after first-line chemotherapy (for patients withEGFR-sensitizing mutations)
Erlotinib
Evidence (erlotinib):
Two randomized placebo-controlled trials indicated that erlotinib prolongs survival and time to deterioration in symptoms in patients with NSCLC after first-line or second-line chemotherapy compared with placebo [11,12] but does not improve survival compared with standard second-line chemotherapy with docetaxel or pemetrexed.[13]
- A trial of erlotinib versus best supportive care included 731 patients; 49% had received two previous chemotherapy regimens, and 93% had received platinum-based chemotherapy.
- OS was 6.7 months among those who had received two previous chemotherapy regimens and 4.7 months among those who had received platinum-based chemotherapy. The HR was 0.70 (P < .001) in favor of erlotinib.[11][Level of evidence A1]
- In a second trial (NCT00556322), which was designed to show the superiority of erlotinib versus standard second-line chemotherapy after disease progression on first-line platinum combination therapy, 424 patients were randomly assigned.
- There was no difference in the primary end point of OS (median OS, 5.3 months vs. 5.5 months; HR, 0.96; 95% CI, 0.78–1.19).[13][Level of evidence A1]
Gefitinib
Evidence (gefitinib):
- A randomized phase III trial evaluated gefitinib versus placebo in 1,692 patients with previously treated NSCLC and showed the following:
- Gefitinib did not improve OS.
- Median survival did not differ significantly between the groups in the overall population (5.6 months for gefitinib and 5.1 months for placebo; HR, 0.89; 95% CI, 0.77–1.02; P = .087) or among the 812 patients with adenocarcinoma (6.3 months vs. 5.4 months; HR, 0.84; CI, 0.68–1.03; P = .089).
- Preplanned subgroup analyses showed significantly longer survival in the gefitinib group than in the placebo group for never-smokers (n = 375; 95% CI, 0.67 [0.49–0.92]; P = .012; median survival 8.9 months vs. 6.1 months) and for patients of Asian origin (n = 342; 95% CI, 0.66 [0.48–0.91]; P = .01; median survival 9.5 months vs. 5.5 months).[14][Level of evidence A1]
- In a large randomized trial, gefitinib was compared with docetaxel in patients with locally advanced or metastatic NSCLC who had been pretreated with platinum-based chemotherapy.[15] The primary objective was to compare OS between the groups with coprimary analyses to assess noninferiority in the overall population and superiority in patients with high EGFR gene copy number in the intention-to-treat population. The 1,466 patients were randomly assigned to receive gefitinib (250 mg per day by mouth; n = 733) or docetaxel (75 mg/m2 intravenously [IV] every 3 weeks; n = 733).
- Noninferiority of gefitinib compared with docetaxel was confirmed for OS (HR, 1.020; 95% CI, 0.905–1.150). However, superiority of gefitinib in patients with high EGFR gene copy number (85 patients vs. 89 patients) was not proven (HR, 1.09; 95% CI, 0.78–1.51; P = .62).
- In the gefitinib group, the most common adverse events were rash or acne (49% vs. 10%) and diarrhea (35% vs. 25%). In the docetaxel group, neutropenia (5% vs. 74%), asthenia (25% vs. 47%), and alopecia (3% vs. 36%) were most common.
- This trial established noninferior survival of patients treated with gefitinib compared with docetaxel, suggesting that gefitinib is a valid treatment for pretreated patients with advanced NSCLC.
Objective response to erlotinib and gefitinib is more likely in patients who have never smoked, are female, are of East Asian race, or have adenocarcinoma or bronchioloalveolar carcinoma.[16,17,18,19,20,21,22] Responses may be associated with the presence of sensitizing mutations in the tyrosine kinase domain of EGFR[17,18,19,21,22] and with the absence of KRAS mutations.[20,21,22][Level of evidence C2] Survival benefit may be greater in patients with EGFR protein expression by immunohistochemistry or increased EGFR gene copy number by fluorescence in situ hybridization studies (FISH),[21,22] but the clinical utility of EGFR testing by immunohistochemistry has been questioned.[23]
Afatinib
Evidence (afatinib):
- Afatinib, an irreversible inhibitor of the ErbB-family of receptors, has been compared with erlotinib as second-line treatment in patients with advanced squamous cell carcinoma. In a randomized, controlled, phase III trial (LUX-Lung 8 [NCT01523587]), patients with stage IIIB/IV squamous cell NSCLC with disease progression after frontline platinum-based chemotherapy were randomly assigned in a 1:1 ratio to receive afatinib (398 patients, 40 mg by mouth every day) or erlotinib (397 patients, 150 mg by mouth every day).[24][Level of evidence B1] The primary end point was PFS. Secondary end points included OS and response rate.
- After a median follow-up of 6.7 months, the PFS was 2.4 months versus 1.9 months (HR, 0.82; 95% CI, 0.68–1.00).
- After a median follow-up of 18.4 months, the median OS was significantly longer in the afatinib arm (7.9 months vs. 6.8 months; HR, 0.81; 95% CI, 0.69–0.95; P = .007). Survival at 6 months (63.6% vs. 54.6%; P = .009), 12 months (36.4% vs. 28.2%; P = .015), and 18 months (22% vs. 14.4%; P = .013) were all significantly better in patients who received afatinib.
- There was no significant difference in response rate between the two arms (6% vs. 3%; P = .551).
- The frequency of adverse events was similar between the two groups with 57% of the patients experiencing a rate of grade 3 or higher adverse events. Grade 3 treatment-related diarrhea and stomatitis occurred more frequently with afatinib; however, grade 3 rash or acne were more common in patients who received erlotinib.
- Afatinib, as compared with erlotinib, represents another option for the second-line treatment of patients with stage IV squamous cell NSCLC.
EGFR-directed therapy (for patients with acquiredEGFRT790M mutations after previous EGFR-directed therapy)
Osimertinib
Evidence (osimertinib):
- An open-label phase III trial (AURA 3 [NCT02151981]) studied osimertinib in patients with NSCLC and EGFR-sensitizing mutations whose disease had progressed after first-line EGFR inhibitors and who had the T790M EGFR resistance mutation as determined by the cobas® EGFR Mutation Test.[25] The trial randomly assigned 419 patients (in a 2:1 ratio) to receive either osimertinib 80 mg by mouth every day or pemetrexed plus carboplatin or cisplatin IV every 3 weeks for up to six cycles; maintenance pemetrexed was allowed for the chemotherapy group. The primary end point was PFS.
- Osimertinib was superior to chemotherapy in prolonging median PFS (10.1 months vs. 4.4 months; HR, 0.30; 95% CI, 0.23–0.41; P < .001).
- The objective response was 71% with osimertinib versus 31% with platinum therapy (odds ratio for objective response, 5.39; 95% CI, 3.47–8.48; P < .001).
- Among 144 patients with central nervous system (CNS) metastases, median PFS duration was 8.5 months with osimertinib versus 4.2 months with platinum therapy (HR, 0.32; 95% CI, 0.21–0.49).
- Adverse events of grade 3 or greater occurred in 23% of osimertinib-treated patients versus 47% of platinum-treated patients.[25][Level of evidence B1]
EGFR-directed therapy after first-line chemotherapy (for patients withEGFRexon 20 insertion mutations)
Amivantamab
Evidence (amivantamab):
- A phase I, open-label, dose-escalation, and dose-expansion study (CHRYSALIS [NCT02609776]) investigated amivantamab, an EGFR-MET bispecific antibody, in several different patient groups. A total of 114 patients with EGFR exon 20 insertion mutations who had received previous platinum-based chemotherapy received the recommended phase II dose of amivantamab (1,050 mg [1,400 mg if >80 kg] IV) once weekly for the first 4 weeks and then once every 2 weeks starting at week 5. The primary end point for the dose-expansion group was overall response rate.[26][Level of evidence C3]
- At the time of clinical data cutoff, 81 patients were evaluable with at least three disease assessments. The overall response rate was 40% (95% CI, 29%–51%), including three complete responses. The median duration of response (DOR) was 11.1 months (95% CI, 6.9–not reached). The clinical benefit rate (including patients with stable disease) was 74% (95% CI, 63%–83%).
- The median PFS was 8.3 months (95% CI, 6.5–10.9); OS data are not mature.
- The most common adverse events were rash (86%), infusion-related reactions (66%), and paronychia (45%). Patients experienced adverse events related to both EGFR inhibition (rash, paronychia, stomatitis, pruritus, diarrhea) and MET inhibition (hypoalbuminemia, peripheral edema). Grade 3 or greater adverse events occurred in 35% of patients, with rash (4%) and infusion reactions (3%) as the most common.
- The infusion reactions occurred primarily with the first exposure to amivantamab. The administration of the first dose was split over 2 days to minimize this toxicity.
The U.S. Food and Drug Administration (FDA) granted accelerated approval to amivantamab for the treatment of patients with EGFR exon 20 mutations whose disease has progressed on or after platinum-based therapy.
Mobocertinib
Evidence (mobocertinib):
- A phase I/II, open-label, dose-escalation, and dose-expansion clinical trial (NCT02716116) examined mobocertinib therapy in multiple molecularly and histologically defined subgroups of patients. The three-part study included a dose-escalation cohort, an expansion cohort in the defined subgroups, and an extension cohort (EXCLAIM). A total of 114 patients with EGFR exon 20 insertion mutations who were previously treated with platinum-containing regimens were treated with mobocertinib (160 mg orally) once daily. The primary end point was overall response, assessed by an independent central review committee.[27][Level of evidence C3]
- The overall response rate was 28% (95% CI, 20%–37%), with a median DOR of 17.5 months (95% CI, 7.4–20.3).
- The median PFS was 7.3 months (95% CI, 5.5–9.2), and the median OS was 24.0 months (95% CI, 14.6–28.8).
- The most common adverse events were diarrhea (91% any grade, 21% grade >3) and rash (45%). Most adverse events were grade 1 to 2 and controlled with dose modification, supportive care, or drug discontinuation. Treatment was discontinued by 17% of participants because of adverse events. One patient died of cardiac failure that was deemed to be treatment related.
The FDA granted accelerated approval to mobocertinib for the treatment of patients with EGFR exon 20 mutations whose disease has progressed on or after platinum-based therapy.
ALK-directed tyrosine kinase inhibitors (TKIs)
ALK-directed TKIs after first-line chemotherapy
Crizotinib
Evidence (crizotinib):
- A study (NCT00585195) that screened 1,500 patients with NSCLC for ALK rearrangements identified 82 patients with advanced ALK-positive disease who were enrolled in a clinical trial that was an expanded cohort study instituted after phase I dose escalation had established a recommended dose of crizotinib dual and ALK inhibitor of 250 mg twice a day in 28-day cycles.[28] Most of the patients had received previous treatment.
- At a mean treatment duration of 6.4 months, the overall response rate was 57% (47 of 82 patients, with 46 confirmed partial responses, and one confirmed complete response); 27 patients (33%) had stable disease.[28][Level of evidence C2]
- The estimated probability of 6-month PFS was 72%.
- The 1-year OS rate was 74% (95% CI, 63%–82%), and the 2-year OS rate was 54% (40%–66%).
- Survival in 30 ALK-positive patients who were given crizotinib in the second-line or third-line setting was significantly longer than in 23 ALK-positive controls identified from a different cohort given any second-line therapy (median OS not reached [95% CI, 14 months–not reached] vs. 6 months [95% CI, 4–17], 1-year OS rate, 70% [95% CI, 50%–83%] vs. 44% [95% CI, 23%–64%], and 2-year OS rate, 55% [33%–72%] vs. 12% [2%–30%]; HR, 0.36; 95% CI, 0.17–0.75; P = .004).[29][Level of evidence C2]
- Common toxicities were grade 1 or 2 (mild) gastrointestinal side effects.
- Patients with ALK rearrangements tended to be younger than those without the rearrangements; most of the patients had little or no exposure to tobacco; and the patients had adenocarcinomas.
- In an open-label, randomized, phase III study, 347 patients with stage IIIB/IV NSCLC-harboring translocations in ALK, who had received one previous regimen of platinum-based chemotherapy, received either crizotinib (250 mg by mouth twice a day) or chemotherapy (pemetrexed 500 mg/m2 if pemetrexed-naïve or docetaxel 75 mg/m2 IV every 21 days).[30]
- The primary end point was PFS. Median PFS was significantly longer in favor of crizotinib (7.7 months vs. 3.0 months, P < .001).[30][Level of evidence B1]
- OS, a secondary end point, was not significantly different, but there was significant crossover in the design.
ALK-directed TKIs after prior ALK TKI therapy
Ceritinib
Evidence (ceritinib):
- A single-arm open-label trial enrolled 163 patients with ALK-translocated stage IIIB/IV NSCLC who had disease progression while receiving crizotinib or were intolerant to the drug.[31] The primary end point was objective response rate according to Response Evaluation Criteria In Solid Tumors (RECIST, version 1.0) with a secondary end point of DOR.
- The objective response rate by blinded independent review was 43.6% (95% CI, 36%–52%), and the median DOR was 7.1 months (range, 5.6–not estimable).[31][Level of evidence C3]
- Of note, 38% of patients required dose modification because of gastrointestinal toxicity; elevation of alanine transaminase to more than five times the upper limit of normal occurred in 27% of patients.
Alectinib
Evidence (alectinib):
- A phase II open-label trial (NCT01871805) enrolled 87 patients with ALK-translocated stage IIIB/IV NSCLC who had disease progression after crizotinib treatment.[32]
- The primary end point was objective response according to RECIST (version 1.1). At the time of primary end point analysis of this ongoing study, 48% of patients (95% CI, 36%–60%) had a confirmed partial response, and 32% had stable disease by blinded independent review. The median DOR was 13.5 months (95% CI, 6.7–not estimable). The estimated median PFS was 8.1 months (95% CI, 6.2–12.6).[32][Level of evidence C3]
- Sixteen patients had measurable CNS disease at baseline, of whom 11 had received prior radiation therapy. The CNS overall response rate was 75% (95% CI, 48%–93%), with 25% of the patients attaining complete response and 50% of the patients attaining partial response.
- The most common side effects were grade 1 or 2 in severity; the most frequent adverse events, occurring in 23% to 36% of patients, were constipation, fatigue, myalgia, and peripheral edema. Dose interruption was needed in 36% of patients, and dose reduction occurred in 16%.
- A second phase II, open-label trial enrolled 138 patients with ALK-positive stage IIIB/IV NSCLC who had disease progression on crizotinib.[33]
- The primary end point was objective response rate by independent central review. The objective response rate was 50% (95% CI, 41%–59%). Median DOR was 11.2 months (95% CI, 9.6–not reached). Median PFS was 8.9 months (95% CI, 5.6–11.3).[33][Level of evidence C3]
- CNS objective response rate in 35 patients with measurable CNS lesions was 57% (95% CI, 39%–74%).
- Common adverse events that were mainly grade 1 or 2, which occurred in 25% to 33% of patients, were constipation, fatigue, and peripheral edema.
Brigatinib
Evidence (brigatinib):
- A phase II open-label trial (NCT02094573) enrolled 222 patients with ALK-translocated locally advanced or metastatic NSCLC who had disease progression after crizotinib treatment. Patients were randomly assigned to receive 90 mg every day (n = 112; 109 treated) or 180 mg every day with a 7-day lead-in at 90 mg every day (n = 110).[34]
- The primary end point assessed by the investigators was objective response rate. The objective response rate was 45% (97.5% CI, 34%–56%) for patients who received the 90 mg dose and 54% (97.5% CI, 43%–65%) for patients who received the 180 mg dose.
- Median PFS was 9.2 months (95% CI, 7.4–15.6) for patients who received the 90 mg dose and 12.9 months (95% CI, 11.1–not reached) for patients who received the 180 mg dose.
- At data cutoff, the median DOR was 13.8 months (95% CI, 5.6–13.8) for patients who received the 90 mg dose and 11.1 months (95% CI, 9.2–13.8) for patients who received the 180 mg dose.[34][Level of evidence B3]
- The CNS objective response rate in patients with measurable CNS lesions was 42% in patients who received 90 mg every day (n = 26) and 67% in patients who received 180 mg every day (n = 18).
- Common adverse events, which were mainly grade 1 or 2 and occurred in 27% to 38% of patients at the higher dose, were nausea, diarrhea, headache, and cough. A subset of pulmonary adverse events with early onset (median onset, day 2) occurred in 14 of 219 treated patients (all grades, 6%; grade ≥3, 3%); none occurred after escalation to 180 mg. These events included dyspnea, hypoxia, cough, pneumonia, or pneumonitis. They were managed with dose interruption. Seven of the 14 patients were successfully retreated with brigatinib.
- The FDA-approved dose of brigatinib is 90 mg every day for 7 days; if tolerated, the dose is increased to 180 mg every day.
Lorlatinib
Evidence (lorlatinib):
- In an open-label ongoing phase II study with multiple cohorts, patients with metastatic ALK-rearranged NSCLC were enrolled into six ALK expansion (EXP) cohorts on the basis of their ALK status and treatment history.[35] They received lorlatinib 100 mg once daily continuously in 21-day cycles. The primary end point was overall and intracranial tumor response by independent central review, as assessed in key pooled subgroups.[35][Level of evidence C3]
- The number of patients treated, the objective response, and the intracranial response rates in each cohort or pooled cohorts are as follows:
- EXP1 (n = 30, treatment naïve).
- Objective response rate, 90.0% (95% CI, 73.5%‒97.9%).
- Intracranial response rate (n = 3), 66.7% (95% CI, 9.4%‒99.2%).
- EXP2 (n = 27, previous crizotinib only) and EXP3A (n = 32, previous crizotinib and chemotherapy).
- Objective response rate, 69.5% (95% CI, 56.1%‒80.8%).
- Intracranial response rate (n = 23), 87.0% (95% CI, 66.4%‒97.2%).
- EXP3B (n = 28, one previous second-generation ALK inhibitor with or without chemotherapy).
- Objective response rate, 32.1% (95% CI, 15.9%‒52.4%).
- Intracranial response rate (n = 9), 55.6% (95% CI, 21.2%‒86.3%).
- EXP4 (n = 65, two previous ALK inhibitors with or without chemotherapy) and EXP5 (n = 46, three previous lines of ALK inhibitors, with or without chemotherapy).
- Objective response rate, 38.7% (95% CI, 29.6%‒48.5%).
- Intracranial response rate (n = 49), 53.1% (95% CI, 38.3%‒67.5%).
- EXP1 (n = 30, treatment naïve).
- The median DOR has not been reached for any of the pooled cohorts.
- The most common adverse event was hypercholesterolemia (16% grade 3–4), and 3% of patients discontinued treatment due to adverse events.
- The number of patients treated, the objective response, and the intracranial response rates in each cohort or pooled cohorts are as follows:
BRAF V600E and MEK inhibitors (for patients withBRAFV600E mutations)
BRAF V600E mutations occur in 1% to 2% of lung adenocarcinomas.
Dabrafenib and trametinib
Evidence (dabrafenib and trametinib):
- In a phase II, multicenter, nonrandomized, open-label study (NCT01336634), 57 patients with progression after at least one to three previous platinum-containing regimens for treatment of metastatic NSCLC, who tested positive for BRAF V600E mutations, were treated with dabrafenib (a BRAF inhibitor) 150 mg twice a day and trametinib (a MEK inhibitor) 2 mg every day.[36]BRAF V600E mutations were ascertained by local testing. The primary end point was investigator-assessed overall response.
- The overall response rate was 63.2% (95% CI, 49.3%–75.6%), as determined independently by investigator and independent review committee assessments. There were 2 out of 36 complete responses by investigator assessment; all responses were deemed partial by the independent review committee.
- The median investigator-assessed PFS was 9.7 months (95% CI, 6.9–19.6 months). The estimated median DOR was 9.0 months (95% CI, 6.9–18.3). The OS data are immature.
- Forty-nine percent of patients had at least one grade 3 or 4 adverse event, the most common of which were neutropenia, hyponatremia, and anemia.[36][Level of evidence C3]
The FDA approved the combination of dabrafenib and trametinib for patients with NSCLC whose tumors harbor BRAF V600E mutations as detected by an FDA-approved test.
ROS1-directed therapy
ROS1 rearrangements occur in approximately 1% of patients with NSCLC.[37] Crizotinib and entrectinib are approved for use in patients with NSCLC with ROS1 rearrangements, with the latter appearing to have greater activity against intracranial disease.
Entrectinib
The FDA approved entrectinib for treatment of patients with metastatic NSCLC whose tumors are ROS1-positive, regardless of the number of previous systemic therapies.
Evidence (entrectinib):
- The safety and clinical activity of entrectinib in ROS1 fusion-positive metastatic NSCLC was determined by integrated analysis of three multicenter, single-arm, open-label clinical trials (ALKA-372-001/EudraCT, 2012-000148-88, STARTRK-1 [NCT02097810], and STARTRK-2 [NCT02568267]).[38] Entrectinib was administered orally at a dose of at least 600 mg once daily. Primary end points were the overall response rate and the DOR determined by blinded independent central review. Of note, time-to-event end points are difficult to interpret in the absence of a control arm. Evaluation of tumor samples for the ROS1 gene fusion was conducted prospectively in local laboratories using either a FISH or next-generation sequencing (NGS) laboratory-developed test.
Seventeen (32%) patients had received no previous systemic therapy, 23 (43%) had received one previous therapy, and 13 (25%) had received two or more lines of treatment. CNS disease was present in 23 (43%) patients at baseline. Thirty-one (59%) patients were never smokers and 52 (98%) patients had adenocarcinoma histology.
- The overall response rate in 53 efficacy-evaluable patients was 77% (95% CI, 64%−88%). Six percent of patients had a complete response and 72% had a partial response. Among patients with CNS disease at baseline, the overall response rate was 74% and all patients had a partial response. Among patients without CNS disease at baseline, the overall response rate was 80% (10% complete response rate; 70% partial response rate). [38][Level of evidence C3]
- The median DOR was 24.6 months (95% CI, 11.4−34.8); 12.6 months (95% CI, 6.5−not estimable) in patients with baseline CNS disease, and 24.6 months (95% CI, 11.4−34.8) in those without CNS disease at baseline.
- Treatment-related adverse events were assessed in 134 patients in the safety-evaluable population. Grade 1 or 2 treatment-related adverse events were observed in 79 (59%) patients. Grade 3 or 4 treatment-related adverse events were observed in 46 (34%) patients. Fifteen (11%) patients had serious treatment-related adverse events. There were no treatment-related deaths.
- The median PFS was 19 months (95% CI, 12.2−36.6); 13.6 months (95% CI, 4.5−not estimable) in patients with baseline CNS disease, and 26.3 months (95% CI, 15.7−36.6) in patients with no baseline CNS disease.
Crizotinib
Crizotinib was approved for patients with metastatic NSCLC whose tumors are ROS1-positive, regardless of the number of previous systemic therapies.
Evidence (crizotinib):
- In an expansion cohort of a phase I study of crizotinib, 50 patients with advanced NSCLC who tested positive for ROS1 rearrangement were treated with oral crizotinib 250 mg twice daily.[39]ROS1 rearrangements were identified using break-apart FISH or reverse transcriptase−polymerase chain reaction assay. Seven patients (14%) had not had any previous treatment for advanced disease, 21 patients (42%) had one previous treatment, and 22 patients (44%) had more than one previous treatment. The primary end point was response rate.
- The overall response rate was 72% (95% CI, 58%–84%). Six percent of patients had a complete response, 66% had a partial response, and 18% had stable disease as their best response.
- Median PFS was 19.2 months (95% CI, 14.4–not reached). The estimated DOR was 17.6 months (95% CI, 14.5–not reached).[39][Level of evidence C3]
- In a phase II, open-label, single-arm trial, 127 East Asian patients with ROS1-positive NSCLC were treated with crizotinib 250 mg twice daily.[40] Twenty-four patients (18.9%) had not had any previous treatment for advanced disease, 53 patients (41.7%) had one previous treatment, and 50 patients (39%) had two or three previous treatments. The primary end point was objective response rate by independent review.
- The objective response rate was 71.7% (95% CI, 63.0%–79.3%). Response rates were similar, irrespective of the number of previous therapies. Complete responses occurred in 13.4% of patients, while 58.3% of patients had partial responses and 16.5% of patients had stable disease as their best response.[40][Level of evidence C3]
- Median PFS was 15.9 months (95% CI, 12.9–24). The DOR was 19.7 months (95% CI, 14.1–not reached).
- OS was 32.5 months (95% CI, 32.5–not reached).
NTRK inhibitors (for patients withNTRKfusions)
Somatic gene fusions in NTRK occur across a range of solid tumors including in fewer than 0.5% of NSCLC tumors.[41,42] These fusions appear to occur more frequently in nonsmokers with lung adenocarcinoma.
Larotrectinib
Evidence (larotrectinib):
- Larotrectinib was studied in three protocols: a phase I study involving adults, a phase I/II study involving children, and a phase II study involving adolescents and adults.[43] Fusions were confirmed in the tumors using either FISH or NGS methods. The primary end point for the combined analysis was objective response rate by independent review and was conducted with input from regulators with the goal of excluding a lower bound of less than 30% for response rate. In total, 55 patients with a median age of 45 years (range, 4 months‒76 years) were enrolled across 17 different NTRK fusion-positive tumor types. All patients had either metastatic disease (82%) or locally advanced unresectable disease (18%). Enrolled patients had received a median of two previous systemic therapies.
- The objective response rate was 75% (95% CI, 61%‒75%) and 73% of these responses lasted at least 6 months.[43][Level of evidence C3]
- Treatment was well tolerated with 93% of adverse events being grade 1 to 2; the most common grade 3 to 4 adverse events were anemia (11% of patients), transaminitis (7%), and neutropenia (7%).
The FDA approved larotrectinib for the treatment of patients who have locally advanced or metastatic tumors that harbor an NTRK gene fusion without a known acquired resistance mutation, and who have no satisfactory alternative treatments or whose cancer has progressed following treatment.
Entrectinib
The FDA granted accelerated approval to entrectinib for the treatment of solid tumors that have an NTRK gene fusion without a known acquired resistance mutation, are metastatic, have progressed after treatment, have no satisfactory alternative therapy, or for cases in which surgical resection is likely to result in severe morbidity.
Evidence (entrectinib):
- The safety and clinical activity of entrectinib in NTRK inhibitor–naïve patients with metastatic or locally-advanced solid tumors (including NSCLC) harboring NTRK1, NTRK2, or NTRK3 gene fusions was determined by integrated analysis of three early-phase, multicenter, single-arm, open-label clinical trials (ALKA-372-001/EudraCT, 2012-000148-88, STARTRK-1 [NCT02097810], and STARTRK-2 [NCT02568267]).[44] Treatment consisted of entrectinib administered orally at a dose of at least 600 mg once per day. The primary end points were objective response and median DOR, which were assessed by blinded independent central review. Of note, time-to-event end points are difficult to interpret in the absence of a control arm. Identification of positive NTRK gene fusion status was conducted prospectively in local laboratories or a central laboratory using various nucleic acid–based tests.
Of 54 patients in the NTRK gene fusion-positive efficacy-evaluable population, 20 (37%) had received no previous systemic therapy, 11 (20%) had received one previous systemic therapy, and 23 (43%) had received two or more systemic therapies. Twelve (22%) patients had CNS disease at baseline. Ten (19%) patients had NSCLC. Fifty-two (96%) patients had an NTRK gene fusion detected by NGS and two (4%) had an NTRK gene fusion detected by other nucleic acid–based tests.
- The objective response rate in 54 patients was 57% (95% CI, 43.2%−70.8%). Seven percent of patients had a complete response and 50% had a partial response. In patients with baseline CNS disease the overall response rate was 50% (all partial responses), whereas in patients without baseline CNS disease, the overall response rate was 60% (10% complete response; 50% partial response).[44][Level of evidence C3]
- The median DOR was 10.4 months (95% CI, 7.1−not estimable). In patients with baseline CNS disease, the DOR was not estimable, whereas it was 12.9 months (95% CI, 7.1−not estimable) in patients with no baseline CNS disease.
- Among 10 patients with NSCLC, the overall response rate was 70% (95% CI, 35%−93%) and the DOR ranged between 1.9 months and 20.1 months. For more information, see the prescribing information.
- The safety-evaluable population consisted of 68 patients with NTRK fusion-positive tumors. Most treatment-related adverse events were grade 1 or 2 and reversible. The most frequent grade 3 or 4 treatment-related adverse events were increased weight gain (10%) and anemia (12%). Serious treatment-related adverse events were reported in 7 (10%) patients. Three (4%) patients had dose interruptions and 27 (40%) patients had dose reductions due to treatment-related adverse events. There were no treatment-related deaths.
- Median PFS was 11.2 months (95% CI, 8.0−14.9). In patients with baseline CNS disease, median PFS was 7.7 months (95% CI, 4.7−not estimable), and it was 12 months (95% CI, 8.7−15.7) in patients with no baseline CNS disease.
RET inhibitors (for patients withRETfusions)
Somatic gene fusions of RET occur in 1% to 2% of patients with NSCLC and in patients with thyroid cancer.[45]
Selpercatinib
Evidence (selpercatinib):
- A phase I/II study (LIBRETTO-001 [NCT03157128]) enrolled patients with RET fusion−positive solid tumors RET fusion status was determined by local molecular testing (NGS, FISH, or polymerase chain reaction assay) without central confirmation. The primary end point was objective response.[46][Level of evidence C3]
- Updated analysis was conducted in 316 patients with RET fusion–positive NSCLC.[46]
- Among the 69 treatment-naïve patients, the objective response rate was 84% (95% CI, 73%–92%), and 6% achieved complete responses. The median DOR was 20.2 months (95% CI, 13.0–could not be evaluated); 40% of responses were ongoing at the data cutoff (median follow-up, 20.3 months). The median PFS was 22.0 months; 35% of patients were alive and progression-free at the data cutoff (median follow-up, 21.9 months).
- Among the 247 patients who had received prior platinum-based chemotherapy, the objective response rate was 61% (95% CI, 55%–67%), and 7% achieved complete responses. The median DOR was 28.6 months (95% CI, 20.4–could not be evaluated); 49% of responses were ongoing (median follow-up, 21.2 months). The median PFS was 24.9 months; 38% of patients were alive and progression-free at the data cutoff (median follow-up, 24.7 months).
- Among the 26 patients with measurable baseline CNS metastasis by the independent review committee, the intracranial objective response rate was 85% (95% CI, 65%–96%), and 27% had complete responses.
- In the full safety population (n = 796), the median treatment duration was 36.1 months.
- There was no significant change in the safety profile. Most adverse events were grade 1 to 2. The most common adverse events were edema, diarrhea, fatigue, dry mouth, hypertension, increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and rash.
The FDA approved selpercatinib to treat adults with locally advanced or metastatic NSCLC with RET gene fusion, as detected by an FDA-approved test.
Pralsetinib
Evidence (pralsetinib):
- A phase I/II study (ARROW [NCT03037385]) enrolled patients with RET fusion−positive solid tumors. Two hundred thirty-three patients had RET fusion−positive NSCLC. RET fusion status was determined by local molecular testing of tumor or circulating tumor nucleic acid (ctDNA) in blood, without central confirmation. The primary end point was objective response.[47][Level of evidence C3]
- Ninety-two patients who had received platinum-based chemotherapy and 29 patients who were treatment-naive (and not candidates for standard platinum-based treatment) received pralsetinib before the efficacy enrollment cutoff (July 11, 2019). Eighty-seven previously treated patients and 27 treatment-naive patients had centrally adjudicated baseline measurable disease, and thus formed the efficacy cohort.
- The overall response rate was 61% (95% CI, 50%–71%) in the 87 patients who had received platinum-based chemotherapy, including complete responses in 6%. The median DOR was not reached (15.2 months–not estimable).
- The overall response rate was 70% (95% CI, 50%–86%) in the 27 treatment-naive patients, including complete responses in 11%. The median DOR was 9.0 months (6.3–not estimable).
- In the 233-patient safety cohort, 93% had treatment-related adverse events, including 48% with grade 3 or worse events. The most common grade 3 or worse treatment-related adverse events were neutropenia (18%), hypertension (11%), and anemia (10%). Dose reductions occurred in 38% of patients, and 6% discontinued treatment because of adverse events.
MET inhibitors (for patients withMETexon 14 skipping mutations)
Dysregulation of the MET proto-oncogene resulting from disruption of distinct splice sites leads to loss of MET exon 14 and enhanced MET signaling. These MET alterations drive tumor proliferation, survival, invasion, and metastasis, and occur in 3% to 4% of patients with NSCLC.[48]
Tepotinib
Evidence (tepotinib):
- An open-label phase II study (VISION [NCT02864992]) enrolled patients with MET exon 14 skipping mutations. The trial included 152 patients who received tepotinib (500 mg orally once daily). MET status was determined centrally either via liquid biopsy (from circulating free DNA obtained from plasma; n = 66) or via tissue biopsy (n = 60). Twenty-seven patients had positive results from both methods. The primary end point was objective response.[49][Level of evidence C3]
- Among the 99 patients who had been followed for at least 9 months (i.e., the efficacy population), the objective response rate as assessed by independent review was 46% (95% CI, 36%–57%), with a median DOR of 11.1 months (95% CI, 7.2 –not estimable). Response rates were similar in the liquid biopsy and tissue biopsy groups.
- Responses were similar regardless of prior therapy.
- Grade 3 or higher adverse events occurred in 28% of patients, including peripheral edema in 7% of patients. Adverse events led to therapy discontinuation in 11% of patients.
Capmatinib
Evidence (capmatinib):
- A phase II study (GEOMETRY [NCT02414139]) evaluated capmatinib (400 mg orally twice daily) in patients with MET exon 14 skipping mutations or MET amplification. MET status was determined centrally. A total of 364 patients were enrolled. The primary end point was overall response.[50][Level of evidence C3]
- Of the 69 patients with MET exon 14 skipping mutations who had received one or two prior lines of therapy, the overall response rate was 41% (95% CI, 29%–53%). The median DOR was 9.7 months (95% CI, 5.6–13.0).
- Of the 28 patients with MET exon 14 skipping mutations who had not received any prior treatment, the overall response rate was 68% (95% CI, 48%–84%). The median DOR was 12.6 months (95% CI, 5.6–not estimable).
- Response rates in patients with MET amplification without the exon 14 skipping mutation did not meet the prespecified threshold for clinically relevant activity.
- Grade 3 to 4 adverse events occurred in 67% of patients. The most common events, regardless of causality, were peripheral edema, nausea, vomiting, and increased creatinine. Adverse events led to therapy discontinuation in 11% of patients.
KRAS G12C inhibitors (for patients withKRASG12C mutations)
Activating mutations in KRAS are found in 25% to 30% of nonsquamous NSCLC, resulting in activation of downstream oncogenic pathways and uncontrolled growth. The G12C single-nucleotide variant, with glycine substituted by cysteine at codon 12, is the most frequent variant in NSCLC, occurring in approximately 13% of lung adenocarcinomas.[51]
Adagrasib
Evidence (adagrasib):
- KRYSTAL-1 (NCT03785249) was a phase I/II multiple expansion cohort clinical trial that investigated adagrasib (600 mg orally twice daily) in patients with advanced solid tumors. A phase II cohort of the trial included patients with NSCLC and KRAS G12C mutations who were previously treated with platinum-based chemotherapy and anti–programmed death 1 (PD-1) or anti–programmed death-ligand 1 (PD-L1) therapy. The cohort enrolled 116 patients; 98.3% had previously received both chemotherapy and immunotherapy. The primary end point was objective response assessed by blinded independent central review.[52][Level of evidence C3]
- Among 112 evaluable patients, the confirmed objective response rate was 42.9% (95% CI, 33.5%–52.6%), including one complete response (0.9%). The median DOR was 8.5 months (95% CI, 6.2–13.8). Disease control occurred in 79.5% of patients (95% CI, 70.8%–86.5%).
- The median PFS was 6.5 months (95% CI, 4.7–8.4), and the median OS was 12.6 months (95% CI, 9.2–19.2).
- Among 33 patients with previously treated stable CNS metastases, the intracranial confirmed objective response rate was 33.3% (95% CI, 18.0%–51.8%).
- Grade 3 or higher adverse events occurred in 44.8% of patients and included two deaths. The most common adverse events were diarrhea, nausea, fatigue, vomiting, dyspnea, increased creatinine, increased ALT, increased AST, and decreased appetite. Adverse events led to therapy discontinuation in 6.9% of patients.
The FDA approved adagrasib for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who received at least one prior systemic therapy.[53]
Sotorasib
Evidence (sotorasib):
- A phase II clinical trial (CodeBreaK 100 [NCT03600883]) evaluated sotorasib (960 mg orally once daily) in patients with KRAS G12C mutations who were previously treated with standard therapies. A total of 126 patients were enrolled. Of 124 evaluable patients, most (81%) received prior platinum-based therapy and inhibitors of PD-1 or PD-L1. The primary end point was overall response according to independent central review.[51][Level of evidence C3]
- For the 124 evaluable patients, the overall response rate was 37.1% (95% CI, 28.6%–46.2%), including complete responses in 3.2%. The median DOR was 11.1 months (95% CI, 6.9–could not be evaluated). Disease control occurred in 80.6% of patients (95% CI, 72.6%–87.2%).
- Median PFS was 6.8 months (95% CI, 5.1–8.2) and median OS was 12.5 months (95% CI, 10.0–could not be evaluated).
- Grade 3 to 4 adverse events occurred in 20.6% of patients. The most common adverse events that were considered treatment related were diarrhea, nausea, increases in ALT or AST, and fatigue. Adverse events led to therapy discontinuation in 7.1% of patients.
The FDA approved sotorasib for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who have received at least one prior systemic therapy.
HER2-targeted therapy (for patients withHER2-mutations)
Mutations in the human epidermal growth factor receptor 2 (HER2) gene are found in 1% to 4% of patients with nonsquamous NSCLC. These mutations are associated with female sex, Asian ethnicity, never-smoking status, a higher incidence of brain metastasis, moderate to poorly differentiated adenocarcinoma histology, and poor prognosis.[54,55]
Trastuzumab deruxtecan
Trastuzumab deruxtecan is an antibody-drug conjugate consisting of a humanized anti-HER2 monoclonal antibody linked to a topoisomerase I inhibitor.
Evidence (trastuzumab deruxtecan):
- DESTINY-Lung01 (NCT03505710) was a multicenter, open-label, two-cohort, phase II clinical trial that investigated trastuzumab deruxtecan at a dose of 6.4 mg/kg of body weight. Enrolled patients had HER2-overexpressing or HER2-mutant unresectable or metastatic nonsquamous NSCLC with disease relapse during standard treatment or with disease refractory to standard treatment. Patients who had previously received a HER2 antibody or an antibody-drug conjugate were ineligible. However, those who had received a HER2 TKI were eligible. The primary end point was objective response as assessed by independent central review. Ninety-one patients with HER2-mutant NSCLC were enrolled; 95% had received previous platinum-based therapy, and 66% had received anti-PD-1 or anti-PD-L1 immunotherapy.[55][Level of evidence C3]
- All 91 patients were evaluable for response. The confirmed objective response rate was 55% (95% CI, 44%–65%), including one complete response (1%). The median DOR was 9.3 months (95% CI, 5.7–14.7). Disease control was observed in 92% of patients (95% CI, 85%–97%).
- The median PFS was 8.2 months (95% CI, 6.0–11.9), and the median OS was 17.8 months (95% CI, 13.8–22.1).
- Among 33 patients with CNS metastases at baseline, 14 had previously received radiation therapy to the brain, and 19 had not. Of these patients, 8 (57%) and 10 (53%), respectively, had a partial response. The median PFS for patients with CNS metastases at baseline was 7.1 months (95% CI, 5.5–9.8), and the median OS was 13.8 months (95% CI, 9.8–20.9).
- Grade 3 or higher drug-related adverse events occurred in 46% of patients. The most common adverse events were nausea, fatigue, alopecia, vomiting, neutropenia, anemia, diarrhea, decreased appetite, leukopenia, and constipation. The most common grade 3 or higher drug-related adverse events were neutropenia (19%) and anemia (10%).
- Drug-related adverse events led to treatment discontinuation in 23 patients (25%) and included pneumonitis in 12 patients (13%) and interstitial lung disease in 5 patients (5%).
- There were two drug-related deaths caused by interstitial lung disease.
The FDA granted accelerated approval to trastuzumab deruxtecan for patients with unresectable or metastatic NSCLC whose tumors have activating HER2 mutations, as detected by an FDA-approved test, and who have received a prior systemic therapy. This approval was based on objective response rate and DOR.
Immunotherapy
Nivolumab is a fully human monoclonal antibody that inhibits the PD-1 coinhibitory immune checkpoint expressed on tumor cells and infiltrating immune cells.[56,57] Pembrolizumab is a humanized monoclonal antibody that inhibits the interaction between the PD-1 coinhibitory immune checkpoint expressed on tumor cells and infiltrating immune cells and its ligands, PD-L1 and PD-L2.[58] Atezolizumab is a PD-L1–blocking antibody.
Nivolumab
Evidence (nivolumab):
In two phase III clinical trials, one conducted in patients with advanced platinum-pretreated squamous NSCLC and the other trial conducted in patients with nonsquamous NSCLC, nivolumab demonstrated a significant improvement in OS compared with the previous standard treatment of docetaxel chemotherapy.[56,57][Level of evidence A1] In addition, the rates of grade 3 and 4 treatment-related toxicity in both trials were significantly lower with nivolumab than with docetaxel. Of note, all patients enrolled in phase III studies of nivolumab had an ECOG performance status of 0 or 1; patients with autoimmune disease, symptomatic interstitial lung disease, or those receiving systemic immunosuppression were excluded from enrollment.
- A randomized, open-label, phase III trial randomly assigned 272 patients with advanced squamous cell NSCLC who had received one regimen of platinum-containing chemotherapy to receive either nivolumab (3 mg/kg every 2 weeks) or docetaxel (75 mg/m2 every 3 weeks), administered until disease progression.[56] The primary end point of this study was OS.
- Nivolumab demonstrated a significant improvement in median OS compared with docetaxel (9.2 months vs. 6 months; P < .001). In addition, the response rate (20% vs. 9%; P = .008) and median PFS (3.5 months vs. 2.8 months; P < .001) favored nivolumab.
- Rates of treatment-related toxicity were significantly lower with nivolumab than with docetaxel (all grades, 58% for nivolumab vs. 86% for docetaxel; grades 3–4, 7% for nivolumab vs. 55% for docetaxel).
- A randomized, open-label, phase III trial included 582 patients with advanced nonsquamous NSCLC who had received one regimen of platinum-containing chemotherapy. Patients were randomly assigned to receive either nivolumab (3 mg/kg every 2 weeks) or docetaxel (75 mg/m2 every 3 weeks), administered until disease progression.[57] Previous maintenance chemotherapy after first-line platinum-doublet was allowed; patients with EGFR mutations or ALK translocations were allowed to have received an additional regimen of therapy with a TKI. The primary end point of this study was OS.
- Patients who received nivolumab had a significant improvement in median OS compared with patients who received docetaxel (12.2 months vs. 9.4 months; HR, 0.73; 96% CI, 0.59–0.89; P = .002). In this study, the response rate (19% vs. 12%; P = .02) but not median PFS (2.3 months for nivolumab vs. 4.2 months for docetaxel) favored nivolumab. The median DOR in patients was 17.2 months for nivolumab and 5.6 months for docetaxel.
- Rates of treatment-related toxicity were significantly lower with nivolumab than with docetaxel (all grades, 69% for nivolumab vs. 88% for docetaxel; grades 3–4, 10% for nivolumab vs. 54% for docetaxel).
Both of these trials demonstrated long-term clinical benefit at the 2-year outcomes. The OS rates for nivolumab at 2 years compared with docetaxel in squamous NSCLC were 23% (95% CI, 16%–30%) versus 8% (95% CI, 4%–13%), and OS rates in nonsquamous NSCLC were 29% (95% CI, 24%–34%) versus 16% (95% CI, 12%–20%).[59] Ongoing responses at 2 years were observed in 10 (37%) confirmed responders with squamous NSCLC and 19 (34%) of 56 responders with nonsquamous NSCLC. No patient treated with docetaxel in either study had an ongoing response.
Nivolumab is now considered a standard second-line therapy for patients with metastatic NSCLC with progression on or after first-line platinum-based chemotherapy and is associated with improved survival and lower rates of toxicity than docetaxel. However, clinical trials of nivolumab to date have not enrolled patients with a history of autoimmune disease, interstitial lung disease, or an ECOG performance status higher than 1. Patients with active autoimmune conditions cannot be treated with nivolumab. Closely monitoring all patients for autoimmune toxicities from treatment is required. Specific algorithms for the management of autoimmune toxicity are included in the FDA label for nivolumab.
Pembrolizumab
Evidence (pembrolizumab):
- In a phase I study with multiple expansion cohorts, pembrolizumab demonstrated significant activity with respect to response rate and DOR.[58][Level of evidence C3]
- In the study, 495 patients received either pembrolizumab 2 mg/kg every 3 weeks, 10 mg/kg every 3 weeks, or 10 mg/kg every 2 weeks. No significant differences were seen among the different treatment schedules. Key exclusion criteria were autoimmune disease, history of pneumonitis, requirement for systemic immunosuppressive therapy, and a performance status higher than 1. The objective response rate was 19.4% (95% CI, 16.0%–23.2%), which included a response rate of 18.0% (95% CI, 14.4%–22.2%) in 394 previously treated patients and 24.8% (95% CI, 16.7%–34.3%) in 101 previously untreated patients. Median PFS was 3.7 months (95% CI, 2.9–4.1) for all patients, 3.0 months (95% CI, 2.2–4.0) for previously treated patients, and 6.0 months (95% CI, 4.1–8.6) for previously untreated patients. The median DOR was 12.5 months (range, 1.0–23.3 months) in all patients.
- The study evaluated the efficacy of pembrolizumab in patients with high levels of PD-L1, as assessed by the anti-PD-L1 antibody clone 22C3. Using the cutoff of membranous staining in at least 50% of tumor cells in a validation group of 73 patients, the response rate was 45.2% (95% CI, 33.5%–57.3%), and the median PFS in this group was 6.3 months (95% CI, 2.9–12.5). Median OS was not reached at the time of publication.
- The estimated prevalence of PD-L1 tumor staining from 1,143 screened patients, of whom 824 had evaluable samples, is as follows: 23.2% had 50% or more tumor cells with staining; 37.6% had between 1% and 49% tumor cells with staining; and 39.2% had less than 1% of tumor cells with staining.
- The most common adverse events were fatigue, pruritus, and decreased appetite. Grade 3 or higher adverse events were reported in 9.5% of patients. Inflammatory and immune-mediated adverse events that occurred in more than 2% of patients were infusion-related reactions (3.0%), hypothyroidism (6.9%), and pneumonitis (3.6%).
- In a phase II/III randomized clinical trial, patients with previously treated NSCLC with PD-L1 expression on at least 1% of tumor cells were randomly assigned (1:1:1) to receive pembrolizumab (2 mg/kg), pembrolizumab (10 mg/kg), or docetaxel (75 mg/m2) every 3 weeks.[60][Level of evidence A1] The primary end points were OS and PFS in the total population and in patients with PD-L1 expression on at least 50% of tumor cells. This study enrolled 1,034 patients; 345 of them were allocated to pembrolizumab (2 mg/kg); 346 were allocated to pembrolizumab (10 mg/kg); and 343 were allocated to docetaxel.
- In the total population, median OS was 10.4 months with pembrolizumab (2 mg/kg), 12.7 months with pembrolizumab (10 mg/kg), and 8.5 months with docetaxel. OS was significantly longer for pembrolizumab (2 mg/kg) versus docetaxel (HR 0.71; 95% CI, 0.58–0.88; P = .0008) and for pembrolizumab (10 mg/kg) versus docetaxel (HR, 0.61; CI, 0.49–0.75; P < .0001).
- In the total population, PFS was not prolonged in the pembrolizumab groups compared with the docetaxel group.
- Among patients with at least 50% of tumor cells expressing PD-L1, OS was significantly longer with pembrolizumab (2 mg/kg) than with docetaxel (median, 14.9 months vs. 8.2 months; HR, 0.54; 95% CI, 0.38–0.77; P = .0002) and with pembrolizumab (10 mg/kg) than with docetaxel (median, 17.3 months vs. 8.2 months; HR, 0.50; CI, 0.36–0.70; P < .0001).
- In the group of patients with at least 50% of tumor cells expressing PD-L1, PFS was significantly longer with pembrolizumab (2 mg/kg) than with docetaxel (median, 5.0 months vs. 4.1 months; HR, 0.59; 95% CI, 0.44–0.78; P = .0001) and with pembrolizumab (10 mg/kg) than with docetaxel (median, 5.2 months vs. 4.1 months; HR, 0.59; CI, 0.45–0.78; P < .0001).
- Grade 3 to 5 treatment-related adverse events were less common with pembrolizumab than with docetaxel (43 [13%] of 339 patients given pembrolizumab (2 mg/kg), 55 [16%] of 343 patients given pembrolizumab (10 mg/kg), and 109 [35%] of 309 patients given docetaxel).
The FDA granted accelerated approval to pembrolizumab as a second-line therapy for patients with NSCLC whose tumors express PD-L1 (>50% staining as determined by an FDA-approved test) with progression on or after first-line chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapies before receiving pembrolizumab (see the FDA label for pembrolizumab).
Atezolizumab
Evidence (atezolizumab):
- Two international, randomized, open-label clinical trials (OAK [NCT02008227] and POPLAR [NCT01903993]) demonstrated efficacy and safety in a total of 1,137 patients with NSCLC who previously received platinum chemotherapy.[61,62][Level of evidence A1] Compared with docetaxel, treatment with atezolizumab in the intended patient population resulted in improved OS rates of 4.2 months in the OAK study and 2.9 months in the POPLAR study.
- In the OAK trial, the median OS was 13.8 months in the atezolizumab arm (95% CI, 11.8–15.7) compared with 9.6 months in the docetaxel arm (95% CI, 8.6–11.2) (HR, 0.74; 95% CI, 0.63–0.87; P = .0004).
- The median OS in the POPLAR trial was 12.6 months in the atezolizumab arm (95% CI, 9.7–16.0) and 9.7 months in the docetaxel arm (95% CI, 8.6–12.0) (HR, 0.69; 95% CI, 0.52–0.92).
- Although the magnitude of improvement correlated with PD-L1 immunohistochemistry expression on tumor cells and tumor-infiltrating immune cells, survival benefit with atezolizumab was seen in patients with tumors with and without PD-L1 expression.
- In the POPLAR trial, the most common (≥20%) adverse reactions were in patients treated with atezolizumab and included fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation.
- The most common (≥2%) grade 3 to 4 adverse events in patients treated with atezolizumab were dyspnea, pneumonia, hypoxia, hyponatremia, fatigue, anemia, musculoskeletal pain, ALT and AST increase, dysphagia, and arthralgia.
- Clinically significant immune-related adverse events for patients who received atezolizumab included pneumonitis, hepatitis, colitis, and thyroid disease.
mTOR inhibitors
Everolimus
Everolimus is used for patients with unresectable, locally advanced or metastatic, progressive, well-differentiated, nonfunctional, neuroendocrine tumors.
Everolimus, an oral mTOR inhibitor, is clinically active against advanced pancreatic and nonpancreatic neuroendocrine tumors.[63] Based on the results of the RADIANT-4 clinical trial,[63] the FDA approved everolimus for the treatment of adult patients with unresectable, locally advanced or metastatic, progressive, well-differentiated (low or intermediate grade), nonfunctional neuroendocrine tumors of lung or gastrointestinal origin.
Evidence (everolimus):
- A randomized, double-blind, placebo-controlled, phase III trial (RADIANT-4 [NCT01524783]) evaluated everolimus in patients older than 18 years with advanced, progressive, well-differentiated, nonfunctional neuroendocrine tumors of lung or gastrointestinal origin.[63] Eligible patients were randomly assigned in a 2:1 ratio to receive everolimus 10 mg daily orally or placebo, both with best supportive care. A total of 302 patients were enrolled (205 in the everolimus arm and 97 in the placebo arm), including 90 patients with neuroendocrine tumors of lung origin (63 in the everolimus arm and 27 in the placebo arm). The primary end point was PFS assessed by central radiology review in the intention-to-treat population.
- Median PFS was 11.0 months in the everolimus arm and 3.9 months in the placebo group (HR, 0.48; 95% CI, 0.35−0.67; P < .00001).[63][Level of evidence B1]
- In a post hoc analysis of the lung subgroup, median PFS by central review was 9.2 months in the everolimus arm and 3.6 months in the placebo arm (HR, 0.50; 95% CI, 0.28−0.88).[63]
- The objective response rate was 2% in patients who received everolimus and 1% in patients who received placebo. Disease stabilization was observed in 81% of patients in the everolimus arm and 64% of patients in the placebo arm.
- The median duration of treatment was longer in the patients who received everolimus compared with those who received placebo (40.4 weeks vs. 19.6 weeks).
- A planned interim analysis of OS showed a 36% reduction in the estimated risk of death with everolimus relative to placebo (HR, 0.64; 95% CI, 0.40−1.05). These results were not statistically significant.
- The most common treatment-related adverse events were stomatitis, diarrhea, fatigue, infections, rash, and peripheral edema. The most common drug-related grade 3 or 4 adverse events were stomatitis, diarrhea, infections, anemia, and fatigue. Grade 3 or 4 adverse events resulted in treatment discontinuation in 12% of patients in the everolimus group and 3% of patients in the placebo group.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Souquet PJ, Chauvin F, Boissel JP, et al.: Polychemotherapy in advanced non small cell lung cancer: a meta-analysis. Lancet 342 (8862): 19-21, 1993.
- Ellis PA, Smith IE, Hardy JR, et al.: Symptom relief with MVP (mitomycin C, vinblastine and cisplatin) chemotherapy in advanced non-small-cell lung cancer. Br J Cancer 71 (2): 366-70, 1995.
- Girling DJ, et al.: Randomized trial of etoposide cyclophosphamide methotrexate and vincristine versus etoposide and vincristine in the palliative treatment of patients with small-cell lung cancer and poor prognosis. Br J Cancer 67 (Suppl 20): A-4;2, 14, 1993.
- Fossella FV, DeVore R, Kerr RN, et al.: Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 18 (12): 2354-62, 2000.
- Shepherd FA, Dancey J, Ramlau R, et al.: Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18 (10): 2095-103, 2000.
- Huisman C, Smit EF, Giaccone G, et al.: Second-line chemotherapy in relapsing or refractory non-small-cell lung cancer: a review. J Clin Oncol 18 (21): 3722-30, 2000.
- Di Maio M, Perrone F, Chiodini P, et al.: Individual patient data meta-analysis of docetaxel administered once every 3 weeks compared with once every week second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 25 (11): 1377-82, 2007.
- Garon EB, Ciuleanu TE, Arrieta O, et al.: Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 384 (9944): 665-73, 2014.
- Hanna N, Shepherd FA, Fossella FV, et al.: Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22 (9): 1589-97, 2004.
- Scagliotti G, Hanna N, Fossella F, et al.: The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist 14 (3): 253-63, 2009.
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al.: Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353 (2): 123-32, 2005.
- Bezjak A, Tu D, Seymour L, et al.: Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 24 (24): 3831-7, 2006.
- Ciuleanu T, Stelmakh L, Cicenas S, et al.: Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 13 (3): 300-8, 2012.
- Thatcher N, Chang A, Parikh P, et al.: Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366 (9496): 1527-37, 2005 Oct 29-Nov 4.
- Kim ES, Hirsh V, Mok T, et al.: Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 372 (9652): 1809-18, 2008.
- Miller VA, Kris MG, Shah N, et al.: Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol 22 (6): 1103-9, 2004.
- Paez JG, Jänne PA, Lee JC, et al.: EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304 (5676): 1497-500, 2004.
- Lynch TJ, Bell DW, Sordella R, et al.: Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350 (21): 2129-39, 2004.
- Pao W, Miller V, Zakowski M, et al.: EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 101 (36): 13306-11, 2004.
- Pao W, Wang TY, Riely GJ, et al.: KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med 2 (1): e17, 2005.
- Tsao MS, Sakurada A, Cutz JC, et al.: Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med 353 (2): 133-44, 2005.
- Hirsch FR, Varella-Garcia M, Bunn PA, et al.: Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol 24 (31): 5034-42, 2006.
- Clark GM, Zborowski DM, Culbertson JL, et al.: Clinical utility of epidermal growth factor receptor expression for selecting patients with advanced non-small cell lung cancer for treatment with erlotinib. J Thorac Oncol 1 (8): 837-46, 2006.
- Soria JC, Felip E, Cobo M, et al.: Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 16 (8): 897-907, 2015.
- Mok TS, Wu Y-L, Ahn M-J, et al.: Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 376 (7): 629-640, 2017.
- Park K, Haura EB, Leighl NB, et al.: Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J Clin Oncol 39 (30): 3391-3402, 2021.
- Zhou C, Ramalingam SS, Kim TM, et al.: Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer: A Phase 1/2 Open-label Nonrandomized Clinical Trial. JAMA Oncol 7 (12): e214761, 2021.
- Kwak EL, Bang YJ, Camidge DR, et al.: Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363 (18): 1693-703, 2010.
- Shaw AT, Yeap BY, Solomon BJ, et al.: Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 12 (11): 1004-12, 2011.
- Shaw AT, Kim DW, Nakagawa K, et al.: Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 368 (25): 2385-94, 2013.
- Shaw AT, Kim DW, Mehra R, et al.: Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 370 (13): 1189-97, 2014.
- Shaw AT, Gandhi L, Gadgeel S, et al.: Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 17 (2): 234-42, 2016.
- Ou SH, Ahn JS, De Petris L, et al.: Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 34 (7): 661-8, 2016.
- Kim DW, Tiseo M, Ahn MJ, et al.: Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 35 (22): 2490-2498, 2017.
- Solomon BJ, Besse B, Bauer TM, et al.: Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 19 (12): 1654-1667, 2018.
- Planchard D, Besse B, Groen HJM, et al.: Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 17 (7): 984-993, 2016.
- Gainor JF, Shaw AT: Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 18 (7): 865-75, 2013.
- Drilon A, Siena S, Dziadziuszko R, et al.: Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol 21 (2): 261-270, 2020.
- Shaw AT, Ou SH, Bang YJ, et al.: Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 371 (21): 1963-71, 2014.
- Wu YL, Yang JC, Kim DW, et al.: Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 36 (14): 1405-1411, 2018.
- Farago AF, Le LP, Zheng Z, et al.: Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 10 (12): 1670-4, 2015.
- Gatalica Z, Xiu J, Swensen J, et al.: Molecular characterization of cancers with NTRK gene fusions. Mod Pathol 32 (1): 147-153, 2019.
- Drilon A, Laetsch TW, Kummar S, et al.: Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 378 (8): 731-739, 2018.
- Doebele RC, Drilon A, Paz-Ares L, et al.: Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 21 (2): 271-282, 2020.
- Drilon A, Hu ZI, Lai GGY, et al.: Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 15 (3): 151-167, 2018.
- Drilon A, Subbiah V, Gautschi O, et al.: Selpercatinib in Patients With RET Fusion-Positive Non-Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. J Clin Oncol 41 (2): 385-394, 2023.
- Gainor JF, Curigliano G, Kim DW, et al.: Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol 22 (7): 959-969, 2021.
- Awad MM, Oxnard GR, Jackman DM, et al.: MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J Clin Oncol 34 (7): 721-30, 2016.
- Paik PK, Felip E, Veillon R, et al.: Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 383 (10): 931-943, 2020.
- Wolf J, Seto T, Han JY, et al.: Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med 383 (10): 944-957, 2020.
- Skoulidis F, Li BT, Dy GK, et al.: Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 384 (25): 2371-2381, 2021.
- Jänne PA, Riely GJ, Gadgeel SM, et al.: Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N Engl J Med 387 (2): 120-131, 2022.
- U.S. Food and Drug Administration: FDA grants accelerated approval to adagrasib for KRAS G12C-mutated NSCLC. Food and Drug Administration, 2022. Available online. Last accessed August 30, 2024.
- Riudavets M, Sullivan I, Abdayem P, et al.: Targeting HER2 in non-small-cell lung cancer (NSCLC): a glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 6 (5): 100260, 2021.
- Li BT, Smit EF, Goto Y, et al.: Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 386 (3): 241-251, 2022.
- Brahmer J, Reckamp KL, Baas P, et al.: Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 373 (2): 123-35, 2015.
- Borghaei H, Paz-Ares L, Horn L, et al.: Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 373 (17): 1627-39, 2015.
- Garon EB, Rizvi NA, Hui R, et al.: Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372 (21): 2018-28, 2015.
- Horn L, Spigel DR, Vokes EE, et al.: Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 35 (35): 3924-3933, 2017.
- Herbst RS, Baas P, Kim DW, et al.: Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387 (10027): 1540-50, 2016.
- Rittmeyer A, Barlesi F, Waterkamp D, et al.: Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389 (10066): 255-265, 2017.
- Fehrenbacher L, Spira A, Ballinger M, et al.: Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387 (10030): 1837-46, 2016.
- Yao JC, Fazio N, Singh S, et al.: Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387 (10022): 968-977, 2016.
Latest Updates to This Summary (08 / 30 / 2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Treatment of Stage IIIA Non-Small Cell Lung Cancer (NSCLC)
Added Osimertinib (for patients with EGFR mutations) as a new subsection.
Treatment of Stages IIIB and IIIC NSCLC
Added Osimertinib (for patients with EGFR mutations) as a new subsection.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of non-small cell lung cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Non-Small Cell Lung Cancer Treatment are:
- Janet Dancey, MD, FRCPC (Ontario Institute for Cancer Research & NCIC Clinical Trials Group)
- Meredith McAdams, MD (National Cancer Institute)
- Monaliben Patel, MD (University of Rochester Medical Center)
- Arun Rajan, MD (National Cancer Institute)
- Eva Szabo, MD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Non-Small Cell Lung Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389304]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us.
Last Revised: 2024-08-30

